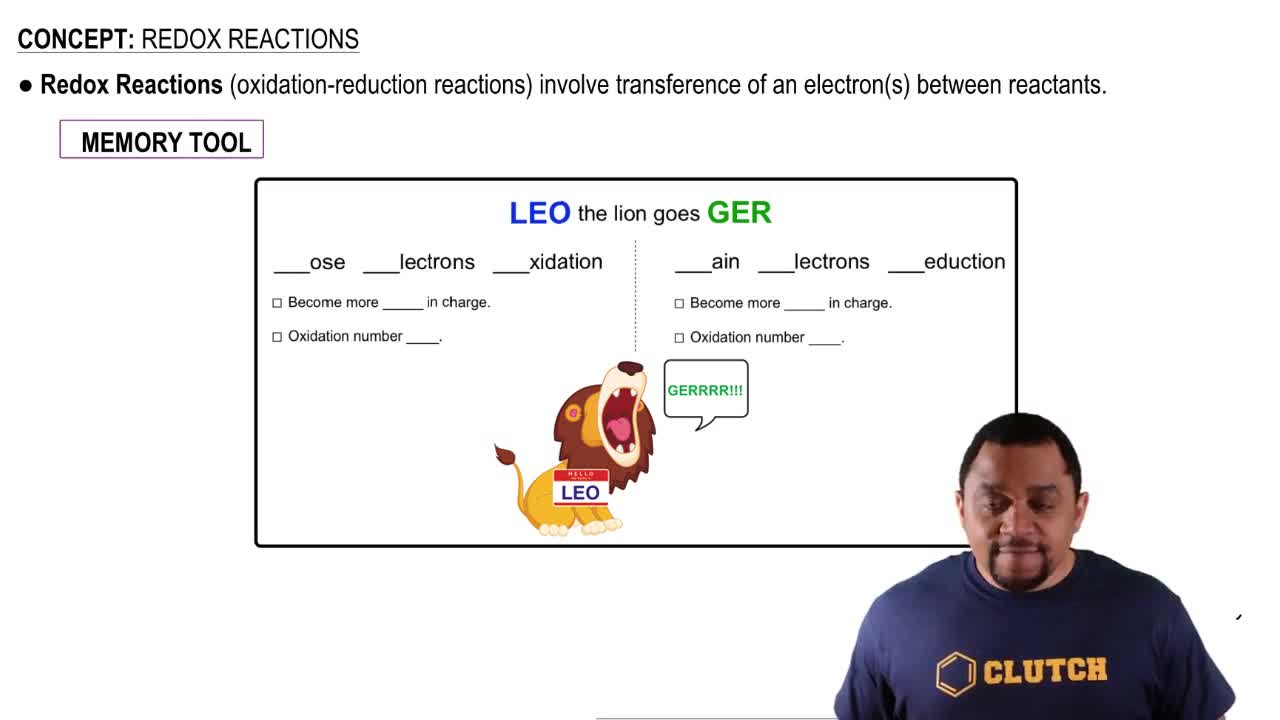
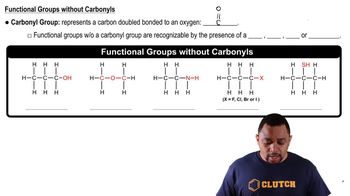
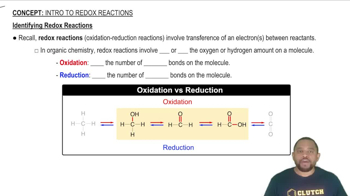
Aqueous solutions of ammonia (NH3) and bleach (active ingredient NaOCl) are sold as cleaning fluids, but bottles of both of them warn: 'Never mix ammonia and bleach, as toxic gases may be produced.' One of the toxic gases that can be produced is chloroamine, NH2Cl. (a) What is the oxidation number of chlorine in bleach? (active ingredient NaOCl) are sold as cleaning fluids, but bottles of both of them warn: “Never mix ammonia and bleach, as toxic gases may be produced.” One of the toxic gases that can be produced is chloroamine, NH2Cl. (b) What is the oxidation number of chlorine in chloramine? (d) Another toxic gas that can be produced is nitrogen trichloride, NCl3. What is the oxidation number of N in nitrogen trichloride?