A voltaic cell utilizes the following reaction: 4 Fe2+1aq2 + O21g2 + 4 H+1aq2 ¡ 4 Fe3+1aq2 + 2 H2O1l2 (b) What is the emf of this cell when 3Fe2+4 = 1.3 M, 3Fe3+4= 0.010 M, PO2 = 0.50 atm, and the pH of the solution in the cathode half-cell is 3.50?

A voltaic cell is constructed with two Zn²⁺/Zn electrodes. The two half-cells have [Zn²⁺] = 1.8 M and [Zn²⁺] = 1.00 × 10⁻² M, respectively. (a) Which electrode is the anode of the cell? (b) What is the standard emf of the cell? (c) What is the cell emf for the concentrations given? (d) For each electrode, predict whether [Zn²⁺] will increase, decrease, or stay the same as the cell operates.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Voltaic Cell and Electrodes
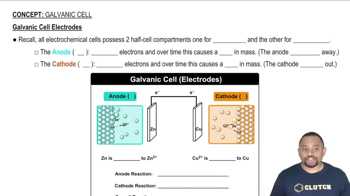
Nernst Equation

Concentration Changes in Electrodes
A voltaic cell utilizes the following reaction: 2 Fe3+1aq2 + H21g2 ¡ 2 Fe2+1aq2 + 2 H+1aq2 (a) What is the emf of this cell under standard conditions?
A voltaic cell utilizes the following reaction: (b) What is the emf for this cell when 3Fe3+4 = 3.50 M, PH2= 0.95 atm, 3Fe2+4 = 0.0010 M, and the pH in both half-cells is 4.00?
A voltaic cell is constructed that is based on the following reaction: Sn2+(aq) + Pb(s) → Sn(s) + Pb2+(aq) (a) If the concentration of Sn2+ in the cathode half-cell is 1.00 M and the cell generates an emf of +0.22 V, what is the concentration of Pb2+ in the anode half-cell?
During a period of discharge of a lead–acid battery, 402 g of Pb from the anode is converted into PbSO4(s). (a) What mass of PbO2(s) is reduced at the cathode during this same period?
During a period of discharge of a lead–acid battery, 402 g of Pb from the anode is converted into PbSO4(s). (b) How many coulombs of electrical charge are transferred from Pb to PbO2?
