The first atoms of seaborgium (Sg) were identified in 1974. The longest-lived isotope of Sg has a mass number of 266. (b) Atoms of Sg are very unstable, and it is therefore difficult to study this element's properties. Based on the position of Sg in the periodic table, what element should it most closely resemble in its chemical properties?

Fill in the blanks in the following table:
Cation Anion Formula Name
Lithium oxide
Fe2+ PO43-
Al2(SO4)3
Copper(II) nitrate
Cr3+ I−
MnClO2
Ammonium carbonate
Zinc perchlorate
Complete the first column of the table.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
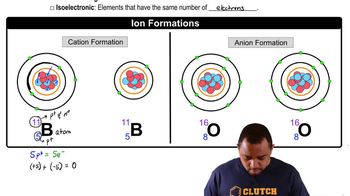
Ionic Compounds

Nomenclature of Ions
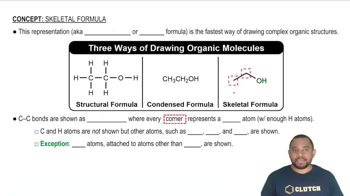
Chemical Formulas
From the molecular structures shown here, identify the one that corresponds to each of the following species: (a) chlorine gas; (b) propane; (c) nitrate ion; (d) sulfur trioxide; (e) methyl chloride, CH3Cl.
Fill in the blanks in the following table:
Cation Anion Formula Name
Lithium oxide
Fe2+ PO43-
Al2(SO4)3
Copper(II) nitrate
Cr3+ I−
MnClO2
Ammonium carbonate
Zinc perchlorate
Complete the third column of the table.
Complete the fourth column of the table.
Elements in the same group of the periodic table often form oxyanions with the same general formula. The anions are also named in a similar fashion. Based on these observations, suggest a chemical formula or name, as appropriate, for each of the following ions: (a) BrO4- (b) SeO32- (c) arsenate ion
Elements in the same group of the periodic table often form oxyanions with the same general formula. The anions are also named in a similar fashion. Based on these observations, suggest a chemical formula or name, as appropriate, for each of the following ions: (d) hydrogen tellurate ion.
