A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (b) After 7.25 mL of base had been added in the titration, the pH was found to be 2.85. What is the Ka for the unknown acid?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 17, Problem 95
Suppose you want to do a physiological experiment that calls for a pH 6.50 buffer. You find that the organism with which you are working is not sensitive to the weak acid H2A 1Ka1 = 2 * 10-2; Ka2 = 5.0 * 10-72 or its sodium salts. You have available a 1.0 M solution of this acid and a 1.0 M solution of NaOH. How much of the NaOH solution should be added to 1.0 L of the acid to give a buffer at pH 6.50? (Ignore any volume change.)
 Verified step by step guidance
Verified step by step guidance1
Identify the relevant equilibrium for the buffer system. Since the desired pH is 6.50, which is closer to the second pKa (7.2), use the second dissociation equilibrium: \( H_2A^- \rightleftharpoons HA^{2-} + H^+ \).
Use the Henderson-Hasselbalch equation for the second dissociation: \( \text{pH} = \text{pKa}_2 + \log \left( \frac{[HA^{2-}]}{[H_2A^-]} \right) \).
Substitute the given pH (6.50) and \( \text{pKa}_2 \) (7.2) into the Henderson-Hasselbalch equation to find the ratio \( \frac{[HA^{2-}]}{[H_2A^-]} \).
Calculate the moles of \( H_2A^- \) initially present in 1.0 L of 1.0 M \( H_2A \), which is 1.0 mol.
Determine the moles of NaOH needed to achieve the desired ratio by using the stoichiometry of the reaction \( H_2A^- + OH^- \rightarrow HA^{2-} + H_2O \), and solve for the volume of 1.0 M NaOH solution required.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Buffer Solutions
A buffer solution is a system that resists changes in pH upon the addition of small amounts of acid or base. It typically consists of a weak acid and its conjugate base or a weak base and its conjugate acid. In this case, the weak acid H2A and its sodium salt will help maintain the desired pH of 6.50 when NaOH is added.
Recommended video:
Guided course
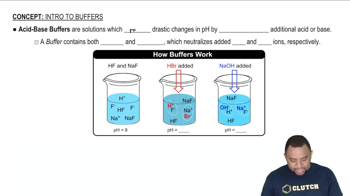
Buffer Solutions
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation relates the pH of a buffer solution to the concentration of its acid and conjugate base. It is expressed as pH = pKa + log([A-]/[HA]), where pKa is the negative logarithm of the acid dissociation constant (Ka). This equation is essential for calculating the required amounts of acid and base to achieve a specific pH.
Recommended video:
Guided course
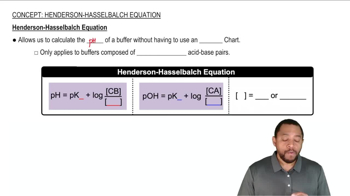
Henderson-Hasselbalch Equation
Acid-Base Neutralization
Acid-base neutralization is a chemical reaction where an acid reacts with a base to produce water and a salt. In this scenario, adding NaOH (a strong base) to the weak acid H2A will neutralize some of the acid, forming its conjugate base and adjusting the pH of the solution. Understanding this reaction is crucial for determining how much NaOH is needed to reach the target pH.
Recommended video:
Guided course
Lewis Acids and Bases
Related Practice
Textbook Question
Textbook Question
Lead(II) carbonate, PbCO3, is one of the components of the passivating layer that forms inside lead pipes. (d) The EPA threshold for acceptable levels of lead ions in water is 15 ppb. Does a saturated solution of lead(II) carbonate produce a solution that exceeds the EPA limit?
Textbook Question
For each pair of compounds, use Ksp values to determine which has the greater molar solubility: (a) CdS or CuS (b) PbCO3 or BaCrO4 (c) Ni(OH)2 or NiCO3 (d) AgI or Ag2SO4.
