Suppose you want to do a physiological experiment that calls for a pH 6.50 buffer. You find that the organism with which you are working is not sensitive to the weak acid H2A 1Ka1 = 2 * 10-2; Ka2 = 5.0 * 10-72 or its sodium salts. You have available a 1.0 M solution of this acid and a 1.0 M solution of NaOH. How much of the NaOH solution should be added to 1.0 L of the acid to give a buffer at pH 6.50? (Ignore any volume change.)

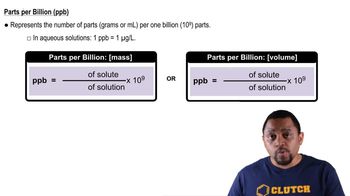
Lead(II) carbonate, PbCO3, is one of the components of the passivating layer that forms inside lead pipes. (d) The EPA threshold for acceptable levels of lead ions in water is 15 ppb. Does a saturated solution of lead(II) carbonate produce a solution that exceeds the EPA limit?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Solubility Product Constant (Ksp)

Lead(II) Carbonate Dissociation
Parts Per Billion (ppb)
For each pair of compounds, use Ksp values to determine which has the greater molar solubility: (a) CdS or CuS (b) PbCO3 or BaCrO4 (c) Ni(OH)2 or NiCO3 (d) AgI or Ag2SO4.
The solubility of CaCO3 is pH dependent. (b) Use the Kb expression for the CO32- ion to determine the equilibrium constant for the reaction CaCO3(s) + H2O(l) ⇌ Ca2+(aq) + HCO3-(aq) + OH-(aq)
Tooth enamel is composed of hydroxyapatite, whose simplest formula is Ca5(PO4)3OH, and whose corresponding 𝐾𝑠𝑝=6.8×10−27. As discussed in the “Chemistry and Life” box on “Tooth Decay and Fluoridation” in Section 17.5, fluoride in fluorinated water or in toothpaste reacts with hydroxyapatite to form fluoroapatite, Ca5(PO4)3F, whose 𝐾𝑠𝑝=1.0×10−60. a. Write the expression for the solubility-constant for hydroxyapatite and for fluoroapatite.
