A sample of 0.2140 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.0950 M NaOH. The acid required 30.0 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 17, Problem 91b
A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (b) After 7.25 mL of base had been added in the titration, the pH was found to be 2.85. What is the Ka for the unknown acid?
 Verified step by step guidance
Verified step by step guidance1
First, calculate the number of moles of NaOH that have been added at the point where the pH is 2.85. This can be done using the formula: moles = Molarity * Volume (in liters). In this case, the molarity of NaOH is 0.1150 M and the volume added is 7.25 mL or 0.00725 L.
Next, calculate the initial number of moles of the unknown acid before any base was added. This can be done using the equivalence point volume of NaOH, which is 15.5 mL or 0.0155 L. The number of moles of acid is equal to the number of moles of base at the equivalence point.
Then, calculate the number of moles of the unknown acid that remain after 7.25 mL of base has been added. This is done by subtracting the moles of base added from the initial moles of acid.
Subsequently, calculate the concentration of the unknown acid that remains after 7.25 mL of base has been added. This is done by dividing the remaining moles of acid by the total volume of the solution at this point (25.0 mL of water + 7.25 mL of base = 32.25 mL or 0.03225 L).
Finally, use the pH and the concentration of the remaining acid to calculate the Ka of the unknown acid. The formula for Ka is: Ka = [H+]*[A-]/[HA], where [H+] is the concentration of hydrogen ions (which can be calculated from the pH), [A-] is the concentration of the base (which is equal to the initial moles of acid minus the remaining moles of acid), and [HA] is the concentration of the remaining acid.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
8mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Monoprotic Acids
Monoprotic acids are acids that can donate only one proton (H+) per molecule during a chemical reaction. This characteristic simplifies the calculation of their dissociation constants, as they only have one equilibrium expression to consider. Understanding the behavior of monoprotic acids is essential for determining their strength and the pH of their solutions.
Recommended video:
Guided course
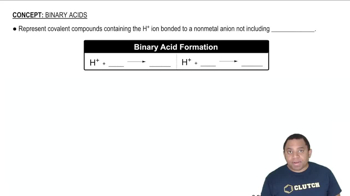
Binary Acids
Titration and Equivalence Point
Titration is a quantitative analytical method used to determine the concentration of a solute in a solution. The equivalence point occurs when the amount of titrant added is stoichiometrically equivalent to the amount of substance being titrated. In this case, it indicates that all the monoprotic acid has reacted with the NaOH, allowing for calculations of the acid's concentration and dissociation constant.
Recommended video:
Guided course
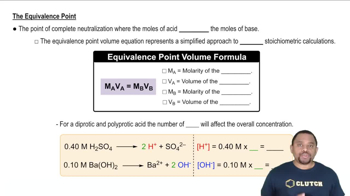
Equivalence Point in Titration
Acid Dissociation Constant (Ka)
The acid dissociation constant (Ka) is a measure of the strength of an acid in solution, defined as the equilibrium constant for the dissociation of the acid into its conjugate base and a proton. It is calculated using the concentrations of the products and reactants at equilibrium. For monoprotic acids, knowing the pH at various points in a titration helps in determining Ka, especially at the half-equivalence point where pH = pKa.
Recommended video:
Guided course
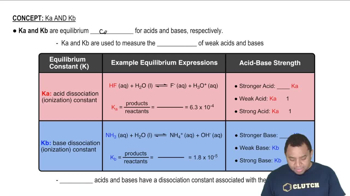
Characteristics of Ka and Kb
Related Practice
Textbook Question
Textbook Question
A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
Textbook Question
Suppose you want to do a physiological experiment that calls for a pH 6.50 buffer. You find that the organism with which you are working is not sensitive to the weak acid H2A 1Ka1 = 2 * 10-2; Ka2 = 5.0 * 10-72 or its sodium salts. You have available a 1.0 M solution of this acid and a 1.0 M solution of NaOH. How much of the NaOH solution should be added to 1.0 L of the acid to give a buffer at pH 6.50? (Ignore any volume change.)
