Two buffers are prepared by adding an equal number of moles of formic acid (HCOOH) and sodium formate (HCOONa) to enough water to make 1.00 L of solution. Buffer A is prepared using 1.00 mol each of formic acid and sodium formate. Buffer B is prepared by using 0.010 mol of each. (b) Which buffer will have the greater buffer capacity?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 17, Problem 91a
A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
 Verified step by step guidance
Verified step by step guidance1
Start by understanding that the equivalence point in a titration is reached when the moles of acid equal the moles of base added. Since the acid is monoprotic, it donates one proton per molecule.
Calculate the moles of NaOH used in the titration. Use the formula: \( \text{moles of NaOH} = \text{volume (L)} \times \text{molarity (M)} \). Convert 15.5 mL to liters by dividing by 1000.
Since the moles of NaOH equal the moles of the unknown acid at the equivalence point, the moles of the acid are the same as the moles of NaOH calculated in the previous step.
Determine the molar mass of the acid using the formula: \( \text{molar mass} = \frac{\text{mass of acid (g)}}{\text{moles of acid}} \). Use the mass of the acid given (0.1687 g) and the moles of acid calculated.
Ensure all units are consistent and check your calculations for accuracy. The molar mass will be in units of g/mol.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Monoprotic Acid
A monoprotic acid is an acid that can donate only one proton (H⁺ ion) per molecule during a chemical reaction. This characteristic simplifies titration calculations, as the amount of base required to neutralize the acid directly relates to the number of moles of the acid present in the solution.
Recommended video:
Guided course
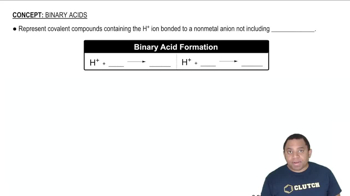
Binary Acids
Titration and Equivalence Point
Titration is a quantitative analytical method used to determine the concentration of a solute in a solution. The equivalence point is reached when the amount of titrant added is stoichiometrically equivalent to the amount of substance being titrated, indicating that the acid has been completely neutralized by the base.
Recommended video:
Guided course
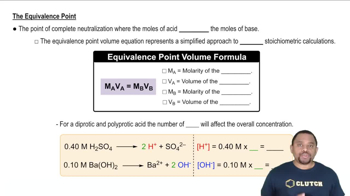
Equivalence Point in Titration
Molar Mass Calculation
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). To calculate the molar mass of the unknown acid, one must first determine the number of moles of acid neutralized at the equivalence point using the volume and concentration of the titrant, and then relate this to the mass of the acid sample.
Recommended video:
Guided course
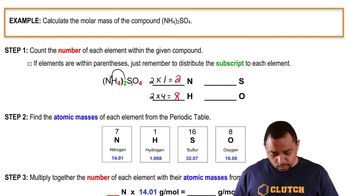
Molar Mass Calculation Example
Related Practice
Textbook Question
1
views
Textbook Question
A sample of 0.2140 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.0950 M NaOH. The acid required 30.0 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
Textbook Question
A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (b) After 7.25 mL of base had been added in the titration, the pH was found to be 2.85. What is the Ka for the unknown acid?
