A buffer contains 0.10 mol of acetic acid and 0.13 mol of sodium acetate in 1.00 L. (a) What is the pH of this buffer?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 17, Problem 28
A buffer contains 0.15 mol of propionic acid (C2H5COOH) and 0.10 mol of sodium propionate (C2H5COONa) in 1.20 L. (a) What is the pH of this buffer? (b) What is the pH of the buffer after the addition of 0.01 mol of NaOH? (c) What is the pH of the buffer after the addition of 0.01 mol of HI?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the components of the buffer system. The buffer consists of propionic acid (C_2H_5COOH) and its conjugate base, sodium propionate (C_2H_5COONa).
Step 2: Use the Henderson-Hasselbalch equation to find the initial pH of the buffer. The equation is pH = pKa + \log\left(\frac{[\text{A}^-]}{[\text{HA}]\right), where [A^-] is the concentration of the conjugate base and [HA] is the concentration of the acid.
Step 3: Calculate the concentrations of propionic acid and sodium propionate in the buffer. Divide the moles of each by the volume of the solution (1.20 L) to find their molar concentrations.
Step 4: For part (b), determine the effect of adding 0.01 mol of NaOH. NaOH will react with the propionic acid, decreasing its concentration and increasing the concentration of the conjugate base. Adjust the concentrations accordingly and use the Henderson-Hasselbalch equation to find the new pH.
Step 5: For part (c), determine the effect of adding 0.01 mol of HI. HI will react with the sodium propionate, decreasing its concentration and increasing the concentration of the propionic acid. Adjust the concentrations accordingly and use the Henderson-Hasselbalch equation to find the new pH.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Buffer Solutions
A buffer solution is a system that resists changes in pH upon the addition of small amounts of acid or base. It typically consists of a weak acid and its conjugate base, which work together to neutralize added acids or bases. In this case, propionic acid and sodium propionate form a buffer that maintains a relatively stable pH.
Recommended video:
Guided course
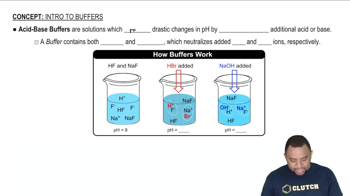
Buffer Solutions
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical formula used to calculate the pH of a buffer solution. It is expressed as pH = pKa + log([A-]/[HA]), where pKa is the negative logarithm of the acid dissociation constant, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid. This equation is essential for determining the pH of the buffer before and after the addition of acids or bases.
Recommended video:
Guided course
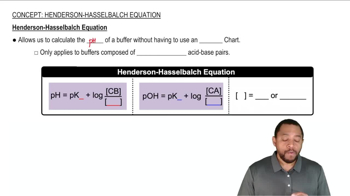
Henderson-Hasselbalch Equation
Effect of Strong Acids and Bases on Buffers
When a strong acid (like HI) or a strong base (like NaOH) is added to a buffer, it can affect the pH depending on the amount added relative to the buffer's capacity. A strong base will react with the weak acid in the buffer, decreasing its concentration and increasing pH, while a strong acid will react with the conjugate base, decreasing its concentration and lowering pH. Understanding these interactions is crucial for predicting pH changes in buffer solutions.
Recommended video:
Guided course

Strong Acid-Strong Base Titration
Related Practice
Textbook Question
Textbook Question
A buffer contains 0.10 mol of acetic acid and 0.13 mol of sodium acetate in 1.00 L. b. What is the pH of the buffer after the addition of 0.020 mol of KOH?
Textbook Question
A buffer contains 0.10 mol of acetic acid and 0.13 mol of sodium acetate in 1.00 L. c. What is the pH of the buffer after the addition of 0.020 mol of HNO3?
Textbook Question
(a) What is the ratio of HCO3- to H2CO3 in blood of pH 7.4?
Textbook Question
(b) What is the ratio of HCO3- to H2CO3 in an exhausted marathon runner whose blood pH is 7.1?
Textbook Question
You have to prepare a pH = 3.50 buffer, and you have the following 0.10 M solutions available: HCOOH, CH3COOH, H3PO4, HCOONa, CH3COONa, and NaH2PO4. Which solutions would you use?
1
views
