(a) What is the ratio of HCO3- to H2CO3 in blood of pH 7.4?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 17, Problem 31a
You have to prepare a pH = 3.50 buffer, and you have the following 0.10 M solutions available: HCOOH, CH3COOH, H3PO4, HCOONa, CH3COONa, and NaH2PO4. Which solutions would you use?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of a buffer solution. A buffer solution resists changes in pH when small amounts of acid or base are added. It typically consists of a weak acid and its conjugate base.
Step 2: Identify the weak acids and their conjugate bases from the given solutions. The weak acids are HCOOH (formic acid), CH3COOH (acetic acid), and H3PO4 (phosphoric acid). Their conjugate bases are HCOONa (sodium formate), CH3COONa (sodium acetate), and NaH2PO4 (sodium dihydrogen phosphate), respectively.
Step 3: Use the Henderson-Hasselbalch equation to determine which buffer system can achieve the desired pH. The equation is: pH = pKa + log([A^-]/[HA]), where [A^-] is the concentration of the conjugate base and [HA] is the concentration of the weak acid.
Step 4: Compare the pKa values of the weak acids to the desired pH of 3.50. The pKa of formic acid (HCOOH) is approximately 3.75, acetic acid (CH3COOH) is approximately 4.76, and the first dissociation of phosphoric acid (H3PO4) is approximately 2.15.
Step 5: Choose the buffer system where the pKa is closest to the desired pH of 3.50. This will provide the most effective buffering capacity around the target pH.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Buffer Solutions
Buffer solutions are mixtures that resist changes in pH when small amounts of acid or base are added. They typically consist of a weak acid and its conjugate base or a weak base and its conjugate acid. Understanding how buffers work is essential for preparing solutions with a specific pH, as they maintain the desired acidity or alkalinity.
Recommended video:
Guided course
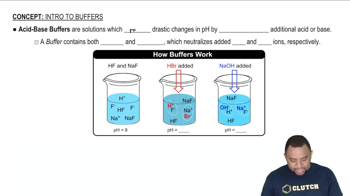
Buffer Solutions
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation relates the pH of a buffer solution to the concentration of its acid and conjugate base. It is expressed as pH = pKa + log([A-]/[HA]), where pKa is the acid dissociation constant. This equation is crucial for determining the appropriate ratio of acid to base needed to achieve a specific pH, such as 3.50 in this case.
Recommended video:
Guided course
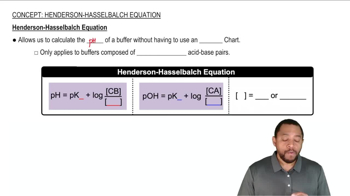
Henderson-Hasselbalch Equation
Weak Acids and Their Conjugate Bases
Weak acids partially dissociate in solution, establishing an equilibrium between the undissociated acid and its ions. The choice of weak acid and its conjugate base is vital for buffer preparation. In this scenario, HCOOH (formic acid) and HCOONa (sodium formate) or CH3COOH (acetic acid) and CH3COONa (sodium acetate) can be used to create a buffer at the desired pH.
Recommended video:
Guided course
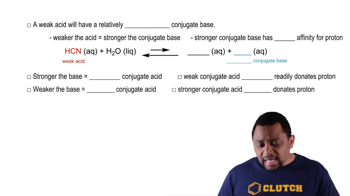
Conjugate Acid-Base Relationships
Related Practice
Textbook Question
Textbook Question
(b) What is the ratio of HCO3- to H2CO3 in an exhausted marathon runner whose blood pH is 7.1?
Textbook Question
You have to prepare a pH = 3.50 buffer, and you have the following 0.10 M solutions available: HCOOH, CH3COOH, H3PO4, HCOONa, CH3COONa, and NaH2PO4. How many milliliters of each solution would you use to make approximately 1 L of the buffer?
Textbook Question
You have to prepare a pH = 5.00 buffer, and you have the following 0.10 M solutions available: HCOOH, HCOONa, CH3COOH, CH3COONa, HCN, and NaCN. Which solutions would you use?
1
views
Textbook Question
You have to prepare a pH = 5.00 buffer, and you have the following 0.10 M solutions available: HCOOH, HCOONa, CH3COOH, CH3COONa, HCN, and NaCN. How many milliliters of each solution would you use to make approximately 1 L of the buffer?
