You are asked to prepare a pH = 4.00 buffer starting from 1.50 L of 0.0200 M solution of benzoic acid 1C6H5COOH2 and any amount you need of sodium benzoate 1C6H5COONa2. (b) How many grams of sodium benzoate should be added to prepare the buffer? Neglect the small volume change that occurs when the sodium benzoate is added.
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 17, Problem 27c
A buffer contains 0.10 mol of acetic acid and 0.13 mol of sodium acetate in 1.00 L. c. What is the pH of the buffer after the addition of 0.020 mol of HNO3?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the components of the buffer system. The buffer consists of acetic acid (CH3COOH) and its conjugate base, sodium acetate (CH3COONa).
Step 2: Write the chemical equation for the reaction between the added HNO3 and the buffer. HNO3 will react with the acetate ion (CH3COO^-) to form acetic acid (CH3COOH) and nitrate ion (NO3^-).
Step 3: Calculate the moles of acetate ion (CH3COO^-) and acetic acid (CH3COOH) after the reaction with HNO3. Subtract the moles of HNO3 from the moles of acetate ion and add the same amount to the moles of acetic acid.
Step 4: Use the Henderson-Hasselbalch equation to find the pH of the buffer. The equation is pH = pKa + log([A^-]/[HA]), where [A^-] is the concentration of acetate ion and [HA] is the concentration of acetic acid.
Step 5: Substitute the values of pKa for acetic acid, and the concentrations of acetate ion and acetic acid into the Henderson-Hasselbalch equation to calculate the pH.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Buffer Solutions
A buffer solution is a system that resists changes in pH upon the addition of small amounts of acid or base. It typically consists of a weak acid and its conjugate base, or a weak base and its conjugate acid. In this case, acetic acid (a weak acid) and sodium acetate (its conjugate base) form a buffer that can maintain pH stability when acids or bases are introduced.
Recommended video:
Guided course
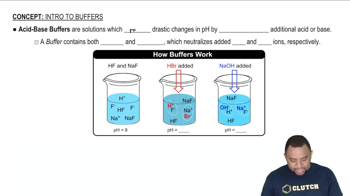
Buffer Solutions
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical formula used to calculate the pH of a buffer solution. It is expressed as pH = pKa + log([A-]/[HA]), where pKa is the negative logarithm of the acid dissociation constant, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid. This equation is essential for determining the pH after the addition of an acid or base.
Recommended video:
Guided course
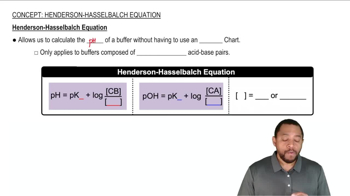
Henderson-Hasselbalch Equation
Acid-Base Neutralization
When an acid is added to a buffer solution, it reacts with the conjugate base present in the buffer, leading to a neutralization reaction. In this scenario, the added HNO3 will react with sodium acetate, converting some of it into acetic acid. This reaction alters the concentrations of the acid and base in the buffer, which must be accounted for when calculating the new pH.
Recommended video:
Guided course
Lewis Acids and Bases
Related Practice
Textbook Question
Textbook Question
A buffer contains 0.10 mol of acetic acid and 0.13 mol of sodium acetate in 1.00 L. (a) What is the pH of this buffer?
Textbook Question
A buffer contains 0.10 mol of acetic acid and 0.13 mol of sodium acetate in 1.00 L. b. What is the pH of the buffer after the addition of 0.020 mol of KOH?
Textbook Question
(a) What is the ratio of HCO3- to H2CO3 in blood of pH 7.4?
Textbook Question
(b) What is the ratio of HCO3- to H2CO3 in an exhausted marathon runner whose blood pH is 7.1?
