Textbook Question
Given that Kb for ammonia is 1.8 × 10-5 and that for hydroxylamine is 1.1 × 10-8, which is the stronger base?

 Verified step by step guidance
Verified step by step guidance
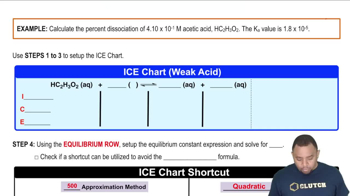
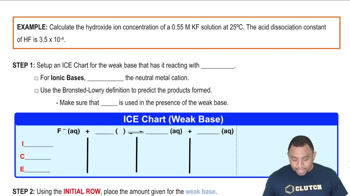

Given that Kb for ammonia is 1.8 × 10-5 and that for hydroxylamine is 1.1 × 10-8, which is the stronger base?
Which is the stronger acid, the ammonium ion or the hydroxylammonium ion?
Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (a) Write out the reaction that leads to this acidic pH.
Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (b) Using Appendix D, calculate the Ka for pyridinium bromide.