Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (a) Write out the reaction that leads to this acidic pH.
Ch.16 - Acid-Base Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 16, Problem 86c
Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (c) A solution of pyridinium bromide has a pH of 2.95. What is the concentration of the pyridinium cation at equilibrium, in units of molarity?
 Verified step by step guidance
Verified step by step guidance1
Identify that pyridinium bromide (C5H5NHBr) is a strong electrolyte and dissociates completely in water into C5H5NH^+ and Br^- ions.
Recognize that the pH of the solution is given as 2.95, which can be used to find the concentration of hydrogen ions [H^+].
Use the formula for pH: \( \text{pH} = -\log[H^+] \) to calculate the concentration of hydrogen ions [H^+].
Since the solution is acidic due to the presence of C5H5NH^+, assume that the concentration of C5H5NH^+ is equal to the concentration of [H^+] at equilibrium.
Conclude that the concentration of the pyridinium cation (C5H5NH^+) at equilibrium is equal to the concentration of [H^+] calculated from the pH.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
6mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Strong Electrolytes
Strong electrolytes are substances that completely dissociate into ions when dissolved in water. This means that in a solution, they exist entirely as ions, which allows them to conduct electricity efficiently. Pyridinium bromide, as a strong electrolyte, dissociates into pyridinium cations (C5H5NH+) and bromide anions (Br-) in aqueous solution.
Recommended video:
Guided course
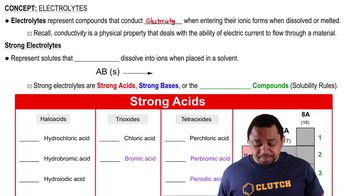
Electrolytes and Strong Acids
pH and Hydrogen Ion Concentration
pH is a measure of the acidity or basicity of a solution, defined as the negative logarithm of the hydrogen ion concentration. A pH of 2.95 indicates a relatively high concentration of hydrogen ions (H+) in the solution, which can be calculated using the formula [H+] = 10^(-pH). This relationship is crucial for determining the concentration of the pyridinium cation in the solution.
Recommended video:
Guided course
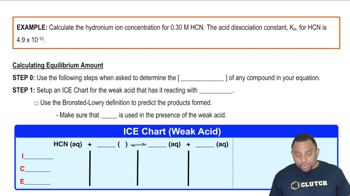
Hydronium Ion Concentration Example
Equilibrium Concentration
Equilibrium concentration refers to the concentration of reactants and products in a chemical reaction at equilibrium. In this context, since pyridinium bromide dissociates completely, the concentration of the pyridinium cation at equilibrium can be inferred from the pH of the solution. By calculating the hydrogen ion concentration and recognizing the stoichiometry of the dissociation, one can determine the molarity of the pyridinium cation.
Recommended video:
Guided course

Thermal Equilibrium
Related Practice
Textbook Question
Textbook Question
Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (b) Using Appendix D, calculate the Ka for pyridinium bromide.
Textbook Question
Predict whether aqueous solutions of the following compounds are acidic, basic, or neutral: (c) Na2CO3
Textbook Question
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (a) AlCl3
Textbook Question
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (b) NaBr
