Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (b) Using Appendix D, calculate the Ka for pyridinium bromide.
Ch.16 - Acid-Base Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 16, Problem 88a
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (a) AlCl3
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the type of compound. AlCl3 is a salt, which is formed from the reaction of an acid and a base. In this case, AlCl3 is formed from the reaction of Al(OH)3, a weak base, and HCl, a strong acid.
Step 2: Determine the potential hydrolysis reactions. Since AlCl3 is a salt, it can undergo hydrolysis, a reaction with water. The potential hydrolysis reactions are Al3+ + H2O -> Al(OH)2+ + H+ and Cl- + H2O -> HCl + OH-.
Step 3: Evaluate the likelihood of the hydrolysis reactions. The Al3+ ion can react with water to produce H+, making the solution acidic. The Cl- ion, however, is the conjugate base of a strong acid (HCl), so it will not react with water to produce OH-.
Step 4: Combine the results of the hydrolysis reactions. Since the Al3+ ion can react with water to produce H+ and the Cl- ion will not react with water to produce OH-, the solution will be acidic.
Step 5: Make the final prediction. Therefore, an aqueous solution of AlCl3 will be acidic.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Base Theory
Acid-base theory explains how substances can donate or accept protons (H+ ions). According to the Brønsted-Lowry theory, acids are proton donors, while bases are proton acceptors. Understanding this concept is crucial for predicting the behavior of substances in solution, particularly how they affect the pH of the solution.
Recommended video:
Guided course
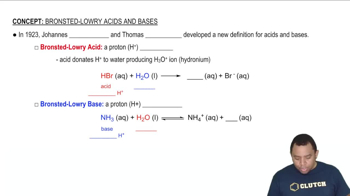
Bronsted-Lowry Acid-Base Theory
Hydrolysis of Salts
When salts dissolve in water, they can undergo hydrolysis, where the ions react with water to produce acidic or basic solutions. For example, cations from weak bases or anions from strong acids can lead to acidic solutions. In the case of AlCl3, the aluminum ion can hydrolyze to form H+ ions, contributing to acidity.
Recommended video:
Guided course
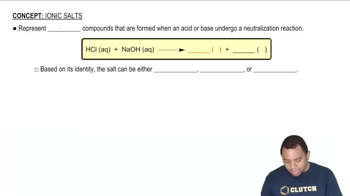
Ionic Salts
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 (very acidic) to 14 (very basic), with 7 being neutral. A solution with a pH less than 7 is considered acidic, while a pH greater than 7 is basic. Understanding the pH scale is essential for predicting the nature of solutions formed by different substances, including salts like AlCl3.
Recommended video:
Guided course
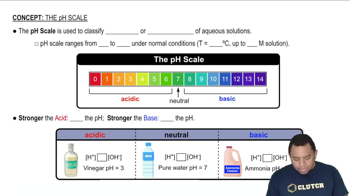
The pH Scale
Related Practice
Textbook Question
Textbook Question
Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (c) A solution of pyridinium bromide has a pH of 2.95. What is the concentration of the pyridinium cation at equilibrium, in units of molarity?
Textbook Question
Predict whether aqueous solutions of the following compounds are acidic, basic, or neutral: (c) Na2CO3
Textbook Question
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (b) NaBr
Textbook Question
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (c) NaClO
1
views
Textbook Question
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (d) 3CH3NH34NO3
