(a) List the following molecules in order of increasing polar- izability: GeCl4, CH4, SiCl4, SiH4, and GeBr4. (b) Predict the order of boiling points of the substances in part (a).
Ch.11 - Liquids and Intermolecular Forces

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 11, Problem 23a
Which member in each pair has the greater dispersion forces? (a) H2O or H2S,
 Verified step by step guidance
Verified step by step guidance1
Identify the type of intermolecular forces present in each molecule. Both H2O and H2S have dispersion forces, but H2O also has hydrogen bonding due to the presence of highly electronegative oxygen.
Consider the molecular size and molar mass. H2S has a larger molar mass than H2O, which generally leads to stronger dispersion forces.
Evaluate the shape and polarizability of the molecules. Larger and more polarizable molecules tend to have stronger dispersion forces.
Compare the overall intermolecular forces. While H2O has strong hydrogen bonds, the question specifically asks about dispersion forces, which are generally stronger in larger molecules like H2S.
Conclude that H2S, being larger and more polarizable, has greater dispersion forces compared to H2O.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Dispersion Forces
Dispersion forces, also known as London dispersion forces, are weak intermolecular forces arising from temporary fluctuations in electron density within molecules. These fluctuations create instantaneous dipoles that induce dipoles in neighboring molecules, leading to an attractive force. The strength of dispersion forces increases with the size and polarizability of the molecules involved.
Recommended video:
Guided course
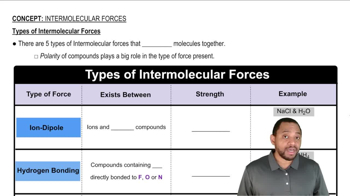
Types of Intermolecular Forces
Molecular Size and Shape
The size and shape of a molecule significantly influence its dispersion forces. Larger molecules have more electrons and a greater surface area, which enhances their polarizability and, consequently, the strength of their dispersion forces. In comparing molecules, those with larger atomic or molecular sizes typically exhibit stronger dispersion forces.
Recommended video:
Guided course

Molecular Shapes and VSEPR
Polarity and Intermolecular Forces
Polarity affects the overall strength of intermolecular forces in a substance. While dispersion forces are present in all molecules, polar molecules like H2O exhibit stronger dipole-dipole interactions compared to nonpolar molecules. Understanding the balance between these forces helps in determining which molecule in a pair has greater dispersion forces, especially when comparing polar and nonpolar substances.
Recommended video:
Guided course

Intermolecular vs Intramolecular Forces
Related Practice
Textbook Question
2
views
Textbook Question
True or false: (b) For the noble gases the dispersion forces decrease while the boiling points increase as you go down the column in the periodic table.
1
views
Textbook Question
True or false: (e) The larger the atom, the more polarizable it is.
1
views
Textbook Question
Which member in each pair has the greater dispersion forces? (b) CO2 or CO, (c) SiH4 or GeH4.
Textbook Question
Which member in each pair has the stronger intermolecular dispersion forces? (a) Br2 or O2 (b) CH3CH2CH2CH2SH or CH3CH2CH2CH2CH2SH (c) CH3CH2CH2Cl or (CH3)2CHCl
Textbook Question
(a) What atoms must a molecule contain to participate in hydrogen bonding with other molecules of the same kind? (b) Which of the following molecules can form hydrogen bonds with other molecules of the same kind: CH3F, CH3NH2, CH3OH, CH3Br?
