Which type of intermolecular force accounts for each of these differences? (a) CH3OH boils at 65 °C; CH3SH boils at 6 °C. (d) Acetone boils at 56 °C, whereas 2-methylpropane boils at -12 °C.
Ch.11 - Liquids and Intermolecular Forces

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 11, Problem 22b
True or false: (b) For the noble gases the dispersion forces decrease while the boiling points increase as you go down the column in the periodic table.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of dispersion forces. Dispersion forces, also known as London dispersion forces, are a type of intermolecular force that exist between atoms and molecules. They are the weakest of the intermolecular forces and occur due to the temporary shifts in the density of the electron cloud of individual atoms and molecules.
Step 2: Understand the concept of boiling points. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a substance is directly related to the strength of the intermolecular forces within the substance.
Step 3: Consider the properties of noble gases. Noble gases are located in the far right column of the periodic table. They are known for their low reactivity due to their full valence electron shells. However, they can still exhibit dispersion forces.
Step 4: Analyze the trend in the periodic table. As you go down the column of noble gases in the periodic table, the size of the atoms increases. This leads to an increase in the strength of the dispersion forces, because larger atoms have more electrons and thus a larger electron cloud, which can lead to greater temporary shifts in electron density.
Step 5: Connect the increase in dispersion forces to boiling points. Since boiling points are directly related to the strength of intermolecular forces, and the dispersion forces are increasing as you go down the column of noble gases, the boiling points of the noble gases also increase as you go down the column in the periodic table.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Noble Gases
Noble gases are a group of elements in Group 18 of the periodic table, known for their lack of reactivity due to having a full valence shell. This group includes helium, neon, argon, krypton, xenon, and radon. As you move down the group, the atomic size increases, which affects the physical properties of these gases, including their boiling points.
Recommended video:
Guided course
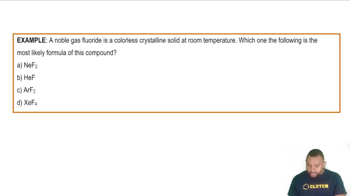
Noble Gas Compounds Example
Dispersion Forces
Dispersion forces, also known as London dispersion forces, are weak intermolecular forces arising from temporary dipoles that occur when electron distributions around atoms fluctuate. These forces increase with the size and polarizability of the atoms or molecules involved. In noble gases, larger atoms have more electrons, leading to stronger dispersion forces as you move down the group.
Recommended video:
Guided course
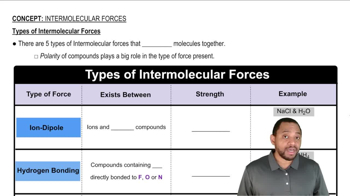
Types of Intermolecular Forces
Boiling Points
The boiling point of a substance is the temperature at which its vapor pressure equals the external pressure, allowing it to transition from liquid to gas. For noble gases, boiling points increase down the group due to the greater strength of dispersion forces, which require more energy to overcome. Thus, while dispersion forces increase, boiling points also rise, contradicting the statement in the question.
Recommended video:
Guided course

Boiling Point Elevation
Related Practice
Textbook Question
2
views
Textbook Question
Which type of intermolecular force accounts for each of these differences? (b) Xe is a liquid at atmospheric pressure and 120 K, whereas Ar is a gas under the same conditions. (c) Kr, atomic weight 84 amu, boils at 120.9 K, whereas Cl2, molecular weight about 71 amu, boils at 238 K.
1
views
Textbook Question
(a) List the following molecules in order of increasing polar- izability: GeCl4, CH4, SiCl4, SiH4, and GeBr4. (b) Predict the order of boiling points of the substances in part (a).
2
views
Textbook Question
True or false: (e) The larger the atom, the more polarizable it is.
1
views
Textbook Question
Which member in each pair has the greater dispersion forces? (a) H2O or H2S,
Textbook Question
Which member in each pair has the greater dispersion forces? (b) CO2 or CO, (c) SiH4 or GeH4.
