Describe the intermolecular forces that must be overcome to convert these substances from a liquid to a gas: (a) SO2, (b) CH3COOH, (c) H2S.

(a) List the following molecules in order of increasing polar- izability: GeCl4, CH4, SiCl4, SiH4, and GeBr4. (b) Predict the order of boiling points of the substances in part (a).
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Polarizability
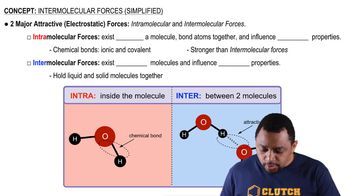
Intermolecular Forces
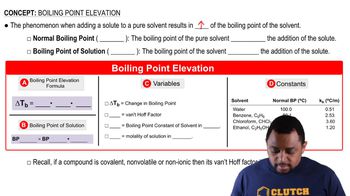
Boiling Point Trends
Which type of intermolecular force accounts for each of these differences? (a) CH3OH boils at 65 °C; CH3SH boils at 6 °C. (d) Acetone boils at 56 °C, whereas 2-methylpropane boils at -12 °C.
Which type of intermolecular force accounts for each of these differences? (b) Xe is a liquid at atmospheric pressure and 120 K, whereas Ar is a gas under the same conditions. (c) Kr, atomic weight 84 amu, boils at 120.9 K, whereas Cl2, molecular weight about 71 amu, boils at 238 K.
True or false: (b) For the noble gases the dispersion forces decrease while the boiling points increase as you go down the column in the periodic table.
True or false: (e) The larger the atom, the more polarizable it is.
Which member in each pair has the greater dispersion forces? (a) H2O or H2S,
