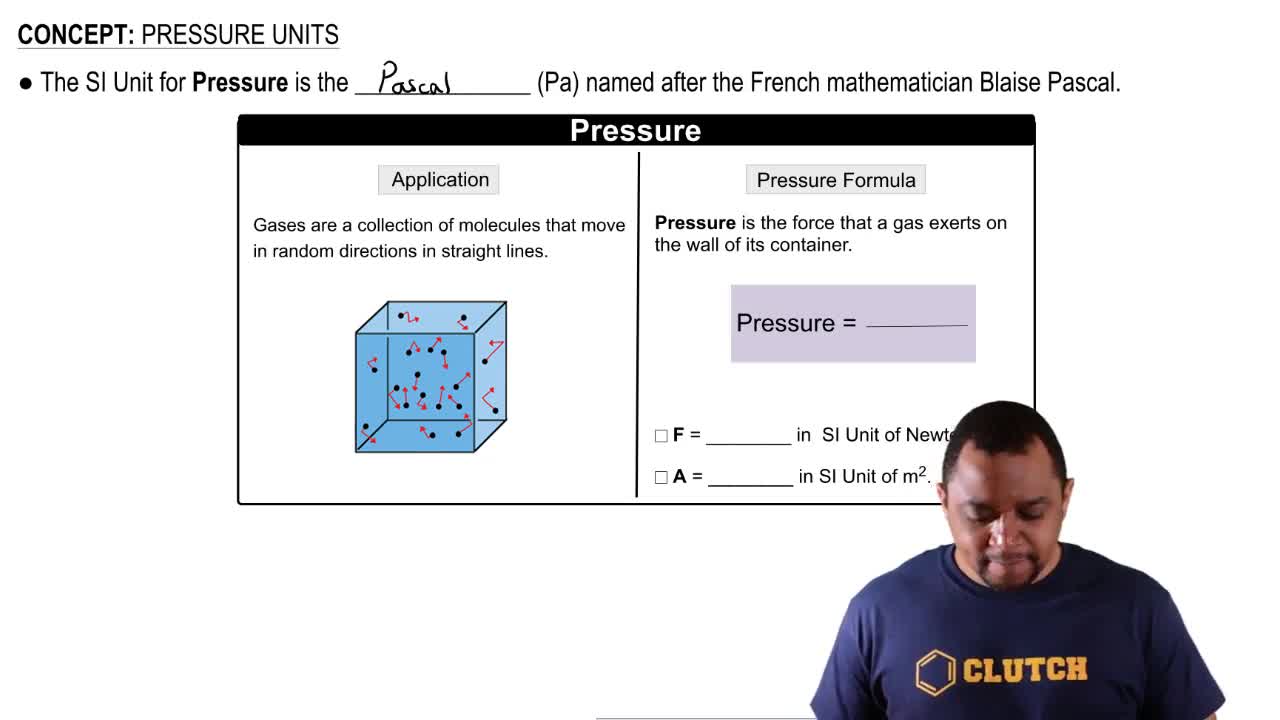
Textbook Question
(a) The compound 1-iodododecane is a nonvolatile liquid with a density of 1.20 g>mL. The density of mercury is 13.6 g>mL. What do you predict for the height of a barometer column based on 1-iodododecane, when the atmospheric pressure is 749 torr?
1
views