How high in meters must a column of glycerol be to exert a pressure equal to that of a 760-mm column of mercury? The density of glycerol is 1.26 g/mL, whereas that of mercury is 13.6 g/mL.

The typical atmospheric pressure on top of Mount Everest (29,032 ft) is about 265 torr. Convert this pressure to c. pascals,
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
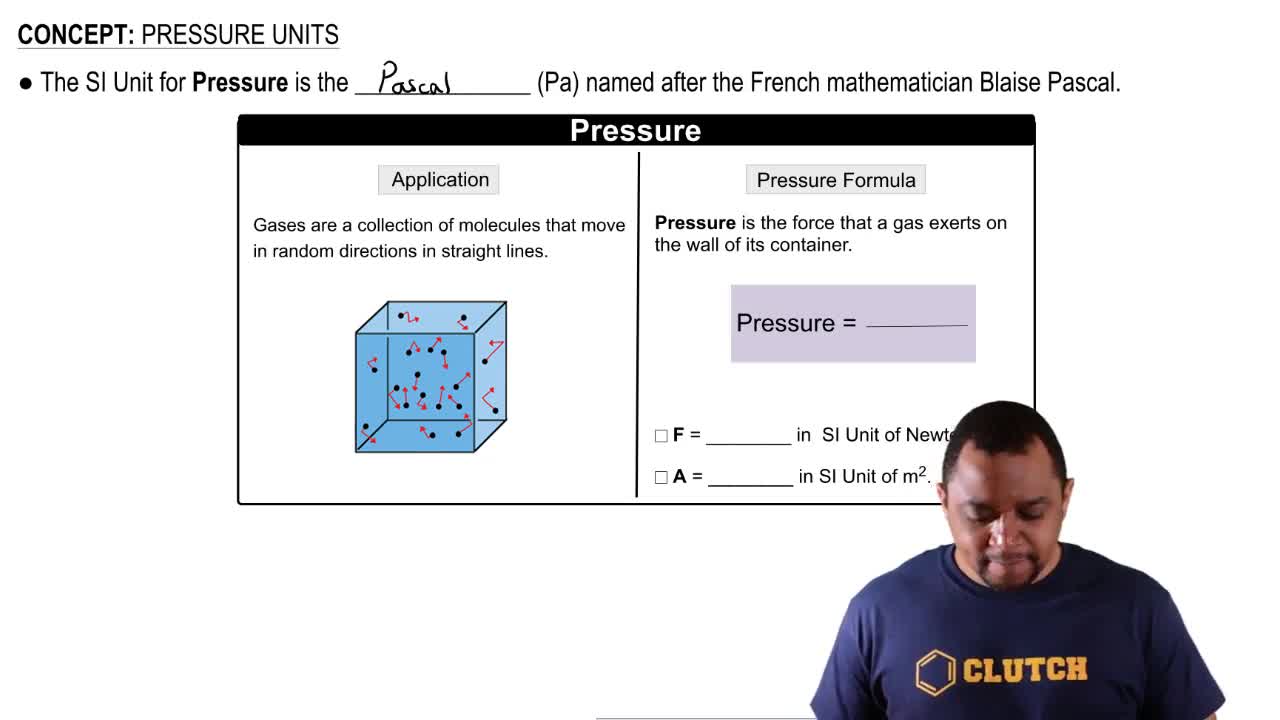
Pressure Units
Conversion Factors

Atmospheric Pressure

What pressure, in atmospheres, is exerted on the body of a diver if they are 15 ft below the surface of the water when the atmospheric pressure is 750 torr? Assume that the density of the water is 1.00 g/cm3=1.00×103 kg/m3. The gravitational constant is 9.81 m/s2, and 1 Pa=1 kg/m-s2.
(a) The compound 1-iodododecane is a nonvolatile liquid with a density of 1.20 g>mL. The density of mercury is 13.6 g>mL. What do you predict for the height of a barometer column based on 1-iodododecane, when the atmospheric pressure is 749 torr?
The typical atmospheric pressure on top of Mount Everest (29,032 ft) is about 265 torr. Convert this pressure to d. bars,
The typical atmospheric pressure on top of Mount Everest (29,032 ft) is about 265 torr. Convert this pressure to e. psi.
Perform the following conversions: (b) 0.685 bar to kilopascals
