Calculate the number of molecules in a deep breath of air whose volume is 2.25 L at body temperature, 37°C, and a pressure of 735 torr.
Ch.10 - Gases

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 10, Problem 38a
If the pressure exerted by ozone, O3, in the stratosphere is 3.0×10−3atm and the temperature is 250 K, how many ozone molecules are in a liter?
 Verified step by step guidance
Verified step by step guidance1
Identify the given values: Pressure (P) = 3.0×10−3 atm, Temperature (T) = 250 K, and Volume (V) = 1 L.
Use the ideal gas law equation, PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant (0.0821 L atm K−1 mol−1), and T is the temperature.
Rearrange the ideal gas law equation to solve for n (number of moles of gas): n = \frac{PV}{RT}.
Calculate the number of moles of ozone using the values of P, V, R, and T substituted into the rearranged ideal gas law equation.
Convert the number of moles of ozone to number of molecules by using Avogadro's number (6.022×10^23 molecules/mol). Multiply the number of moles by Avogadro's number to find the total number of ozone molecules in 1 liter.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. In this context, P is the pressure of ozone, V is the volume (1 liter), n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin. This law allows us to calculate the number of molecules present in a given volume of gas under specific conditions.
Recommended video:
Guided course

Ideal Gas Law Formula
Avogadro's Number
Avogadro's Number, approximately 6.022 × 10²³, is the number of particles (atoms, molecules, etc.) in one mole of a substance. This concept is crucial for converting between moles and molecules. Once we determine the number of moles of ozone using the Ideal Gas Law, we can multiply by Avogadro's Number to find the total number of ozone molecules in the specified volume.
Recommended video:
Guided course
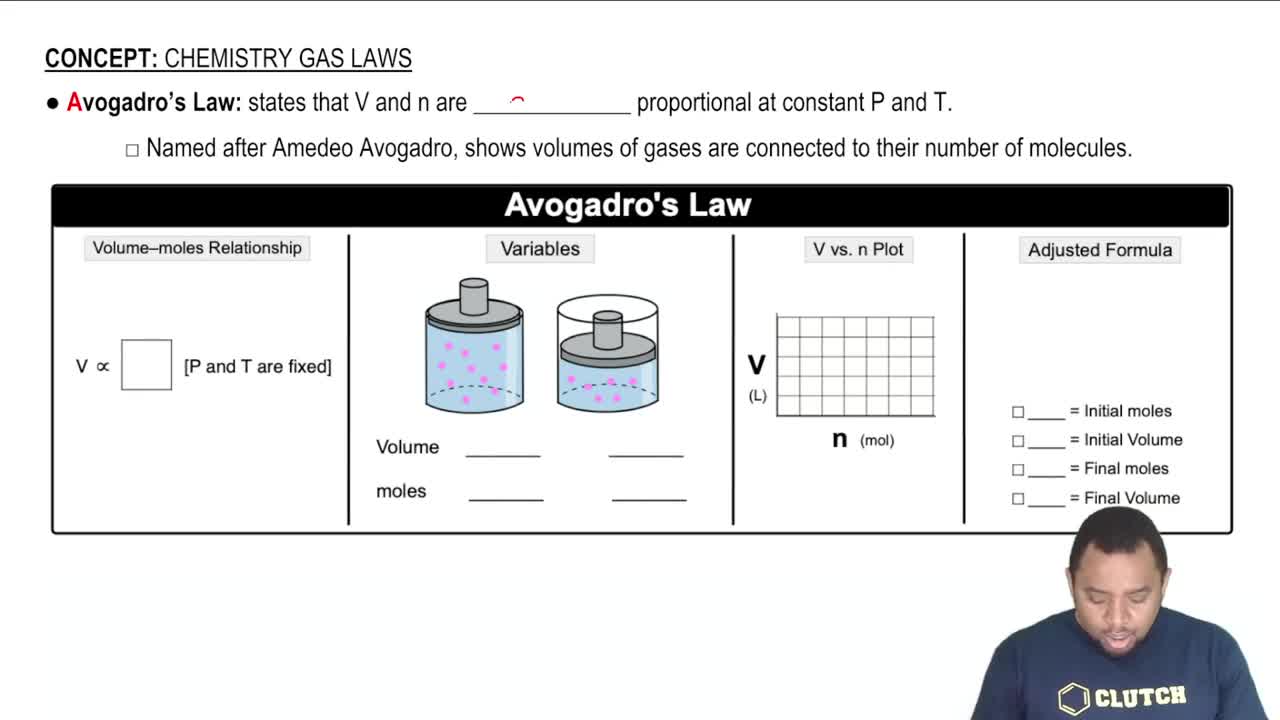
Avogadro's Law
Gas Pressure and Temperature Relationship
Gas pressure is influenced by temperature, as described by the kinetic molecular theory. Higher temperatures increase the kinetic energy of gas molecules, leading to higher pressure if the volume is constant. In this problem, understanding how pressure and temperature interact is essential for applying the Ideal Gas Law correctly to find the number of ozone molecules at the given conditions.
Recommended video:
Guided course
Standard Temperature and Pressure
Related Practice
Textbook Question
Textbook Question
The adult blue whale has a lung capacity of 5.0×103 L. Calculate the mass of air (assume an average molar mass of 28.98 g/mol) contained in an adult blue whale’s lungs at 0.0°C and 1.00 atm, assuming the air behaves ideally.
Textbook Question
Carbon dioxide makes up approximately 0.04% of Earth’s atmosphere. If you collect a 2.0-L sample from the atmosphere at sea level (1.00 atm) on a warm day (27°C), how many CO2 molecules are in your sample?
Textbook Question
A scuba diver’s tank contains 0.29 kg of O2 compressed into a volume of 2.3 L. b. What volume would this oxygen occupy at 26°C and 0.95 atm?
Textbook Question
An aerosol spray can with a volume of 250 mL contains 2.30 g of propane gas (C3H8) as a propellant. a. If the can is at 23°C, what is the pressure in the can?
