Textbook Question
The adult blue whale has a lung capacity of 5.0×103 L. Calculate the mass of air (assume an average molar mass of 28.98 g/mol) contained in an adult blue whale’s lungs at 0.0°C and 1.00 atm, assuming the air behaves ideally.

 Verified step by step guidance
Verified step by step guidance

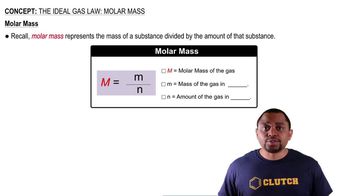
The adult blue whale has a lung capacity of 5.0×103 L. Calculate the mass of air (assume an average molar mass of 28.98 g/mol) contained in an adult blue whale’s lungs at 0.0°C and 1.00 atm, assuming the air behaves ideally.
If the pressure exerted by ozone, O3, in the stratosphere is 3.0×10−3atm and the temperature is 250 K, how many ozone molecules are in a liter?
Carbon dioxide makes up approximately 0.04% of Earth’s atmosphere. If you collect a 2.0-L sample from the atmosphere at sea level (1.00 atm) on a warm day (27°C), how many CO2 molecules are in your sample?