(a) Amonton's law expresses the relationship between pressure and temperature. Use Charles's law and Boyle's law to derive the proportionality relationship between P and T.

(d) If you measure pressure in bars instead of atmospheres, calculate the corresponding value of R in L-bar/mol-K.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
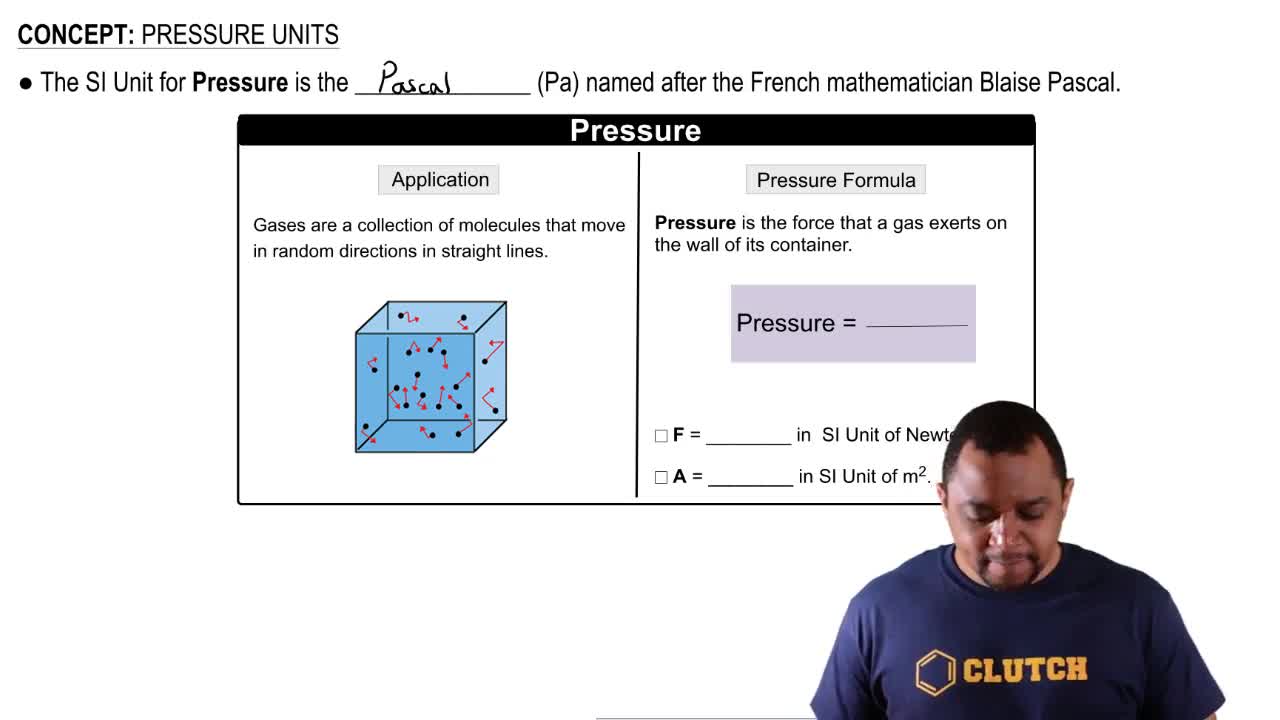
Key Concepts
Ideal Gas Law

Units of Pressure
Ideal Gas Constant (R)

(b) What is the molar volume of an ideal gas at STP?
Suppose you are given two 1-L flasks and told that one contains a gas of molar mass 30 and the other a gas of molar mass 60, both at the same temperature. The pressure in flask A is x atm, and the mass of gas in the flask is 1.2 g. The pressure in flask B is 0.5x atm, and the mass of gas in that flask is 1.2 g. Which flask contains the gas of molar mass 30, and which contains the gas of molar mass 60?
Suppose you are given two flasks at the same temperature, one of volume 2 L and the other of volume 3 L. The 2-L flask contains 4.8 g of gas, and the gas pressure is x atm. The 3-L flask contains 0.36 g of gas, and the gas pressure is 0.1x. Do the two gases have the same molar mass? If not, which contains the gas of higher molar mass?
Complete the following table for an ideal gas:
