(d) If you measure pressure in bars instead of atmospheres, calculate the corresponding value of R in L-bar/mol-K.
Ch.10 - Gases

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 10, Problem 33
Complete the following table for an ideal gas:
 Verified step by step guidance
Verified step by step guidance1
Identify the known variables provided in the problem for each row of the table, such as pressure (P), volume (V), number of moles (n), and temperature (T).
Use the ideal gas law equation, PV = nRT, where R is the ideal gas constant. If any variable is missing, rearrange the equation to solve for the missing variable.
Convert all units to the appropriate SI units: pressure in pascals (Pa), volume in cubic meters (m^3), temperature in Kelvin (K), and the amount of substance in moles (mol).
Plug the known values into the rearranged ideal gas law equation to solve for the unknown variable. Ensure that all units are consistent to avoid errors in calculation.
Check the completed table for consistency and accuracy in the values calculated, ensuring that they adhere to the behavior expected from an ideal gas under the given conditions.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law is a fundamental equation in chemistry that relates the pressure, volume, temperature, and number of moles of an ideal gas. It is expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin. This law helps predict the behavior of gases under various conditions.
Recommended video:
Guided course

Ideal Gas Law Formula
Gas Properties
Gases have unique properties that distinguish them from solids and liquids, including low density, compressibility, and the ability to fill their container. The behavior of gases can be described using various laws, such as Boyle's Law (pressure-volume relationship) and Charles's Law (volume-temperature relationship), which are essential for understanding how gases respond to changes in conditions.
Recommended video:
Guided course
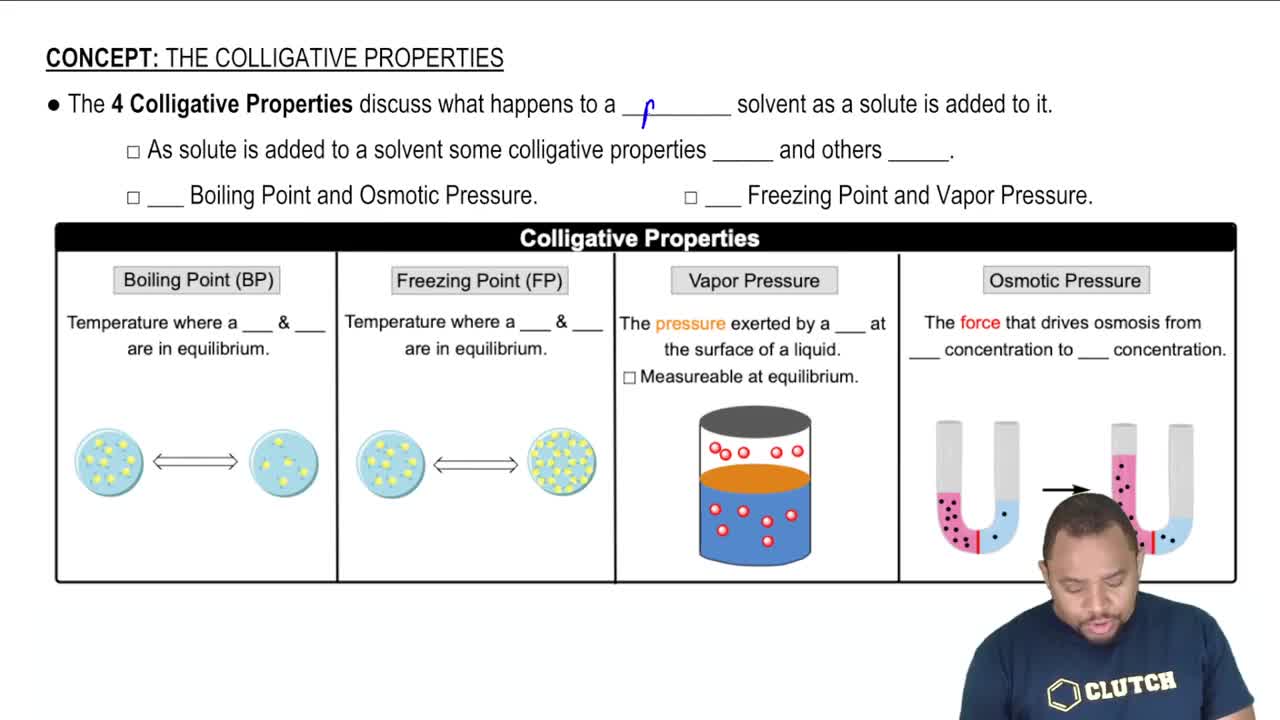
Colligative Properties
Kinetic Molecular Theory
The Kinetic Molecular Theory explains the behavior of gases at a molecular level, positing that gas particles are in constant, random motion and that their collisions with each other and the walls of their container are elastic. This theory helps to explain gas pressure as a result of particle collisions and provides insight into temperature as a measure of the average kinetic energy of the gas particles.
Recommended video:
Guided course

Kinetic Molecular Theory
Related Practice
Textbook Question
Textbook Question
Suppose you are given two 1-L flasks and told that one contains a gas of molar mass 30 and the other a gas of molar mass 60, both at the same temperature. The pressure in flask A is x atm, and the mass of gas in the flask is 1.2 g. The pressure in flask B is 0.5x atm, and the mass of gas in that flask is 1.2 g. Which flask contains the gas of molar mass 30, and which contains the gas of molar mass 60?
Textbook Question
Suppose you are given two flasks at the same temperature, one of volume 2 L and the other of volume 3 L. The 2-L flask contains 4.8 g of gas, and the gas pressure is x atm. The 3-L flask contains 0.36 g of gas, and the gas pressure is 0.1x. Do the two gases have the same molar mass? If not, which contains the gas of higher molar mass?
