Suppose you are given two 1-L flasks and told that one contains a gas of molar mass 30 and the other a gas of molar mass 60, both at the same temperature. The pressure in flask A is x atm, and the mass of gas in the flask is 1.2 g. The pressure in flask B is 0.5x atm, and the mass of gas in that flask is 1.2 g. Which flask contains the gas of molar mass 30, and which contains the gas of molar mass 60?
Ch.10 - Gases

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 10, Problem 34
Calculate each of the following quantities for an ideal gas: (a) the volume of the gas, in liters, if 1.50 mol has a pressure of 126.7 kPa at a temperature of -6 °C, (b) the absolute temperature of the gas at which 3.33 * 10^-3 mol occupies 478 mL at 99.99 kPa, (c) the pressure, in pascals, if 0.00245 mol occupies 413 mL at 138 °C.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the ideal gas law equation: PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin.
Step 2: For part (a), convert the given temperature from Celsius to Kelvin by adding 273.15 to the Celsius temperature. Use the ideal gas constant R = 8.314 J/(mol·K) and rearrange the ideal gas law to solve for volume V: V = nRT/P.
Step 3: For part (b), convert the given volume from milliliters to liters by dividing by 1000. Rearrange the ideal gas law to solve for temperature T: T = PV/(nR). Ensure the pressure is in the correct units (Pa) by converting kPa to Pa (1 kPa = 1000 Pa).
Step 4: For part (c), convert the given temperature from Celsius to Kelvin. Convert the volume from milliliters to liters. Rearrange the ideal gas law to solve for pressure P: P = nRT/V. Ensure the final pressure is in pascals.
Step 5: Substitute the known values into the rearranged equations for each part and solve for the unknown quantity, ensuring all units are consistent throughout the calculations.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law is a fundamental equation in chemistry that relates the pressure, volume, temperature, and number of moles of an ideal gas. It is expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is the absolute temperature in Kelvin. This law allows for the calculation of one variable when the others are known, making it essential for solving problems involving gases.
Recommended video:
Guided course

Ideal Gas Law Formula
Absolute Temperature
Absolute temperature is a temperature measurement on the Kelvin scale, which starts at absolute zero (0 K), the point at which molecular motion ceases. To convert Celsius to Kelvin, one adds 273.15 to the Celsius temperature. Understanding absolute temperature is crucial in gas calculations, as the Ideal Gas Law requires temperature to be in Kelvin to ensure accurate results.
Recommended video:
Guided course
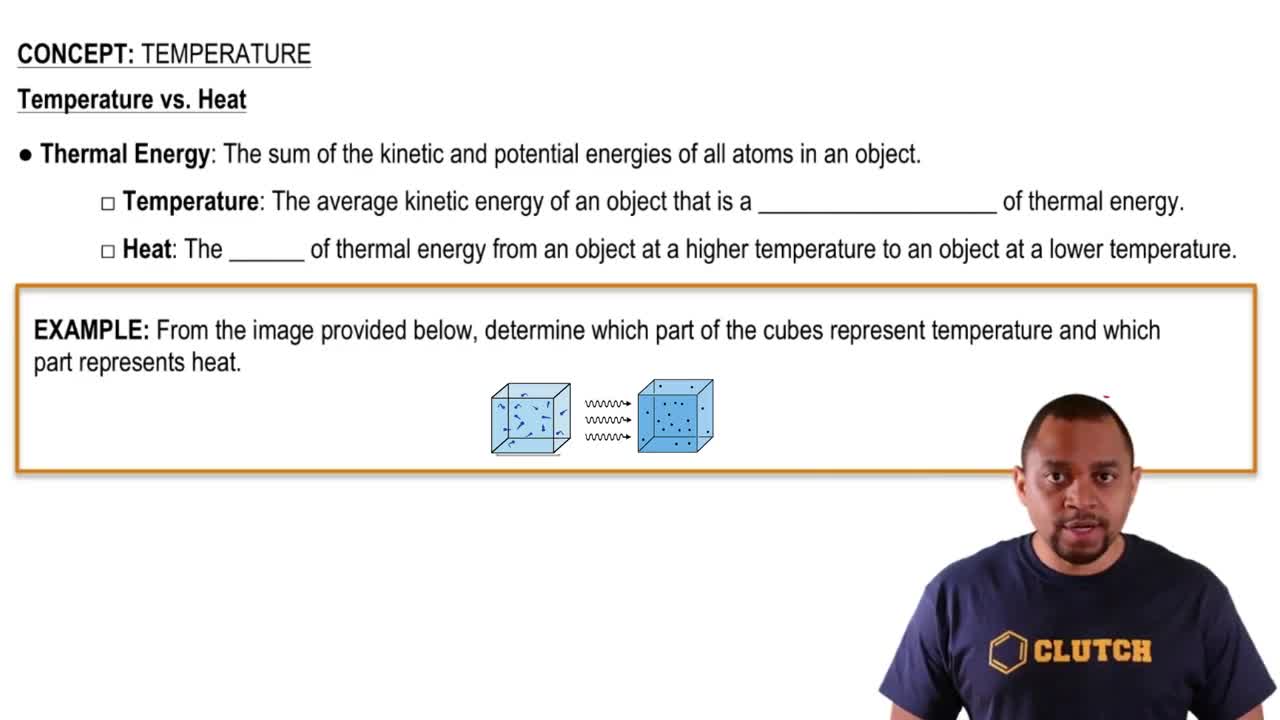
Temperature vs Heat
Units of Measurement
In gas calculations, it is important to use consistent units for pressure, volume, and temperature. Pressure can be measured in pascals (Pa) or kilopascals (kPa), volume in liters (L) or milliliters (mL), and temperature in Kelvin (K). Converting between these units is often necessary to apply the Ideal Gas Law correctly, ensuring that all variables are compatible for accurate calculations.
Recommended video:
Guided course
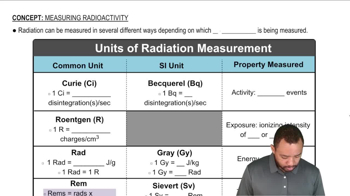
Units of Radiation Measurement
Related Practice
Textbook Question
Textbook Question
Suppose you are given two flasks at the same temperature, one of volume 2 L and the other of volume 3 L. The 2-L flask contains 4.8 g of gas, and the gas pressure is x atm. The 3-L flask contains 0.36 g of gas, and the gas pressure is 0.1x. Do the two gases have the same molar mass? If not, which contains the gas of higher molar mass?
Textbook Question
Complete the following table for an ideal gas:
Textbook Question
Calculate the number of molecules in a deep breath of air whose volume is 2.25 L at body temperature, 37°C, and a pressure of 735 torr.
