The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (a) What is the maximum wavelength of photons that can cause C-Br bond dissociation?
Ch.18 - Chemistry of the Environment

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 18, Problem 18
In CF3Cl, the C-Cl bond dissociation energy is 339 kJ/mol. In CCl4, the C-Cl bond dissociation energy is 293 kJ/mol. What is the range of wavelengths of photons that can cause C-Cl bond rupture in one molecule but not in the other?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of bond dissociation energy, which is the energy required to break a bond in a molecule. Here, we have two different C-Cl bonds with different dissociation energies: 339 kJ/mol for CF3Cl and 293 kJ/mol for CCl4.
Step 2: Convert the bond dissociation energies from kJ/mol to energy per photon. Use the formula: Energy per photon (J) = (Bond dissociation energy (kJ/mol) * 1000 J/kJ) / Avogadro's number (6.022 x 10^23 mol^-1).
Step 3: Calculate the frequency of the photon required to break the bond using the energy per photon. Use the formula: Frequency (ν) = Energy per photon (J) / Planck's constant (6.626 x 10^-34 J·s).
Step 4: Convert the frequency to wavelength using the speed of light. Use the formula: Wavelength (λ) = Speed of light (c, approximately 3.00 x 10^8 m/s) / Frequency (ν).
Step 5: Determine the range of wavelengths that can break the C-Cl bond in one molecule but not the other by comparing the wavelengths calculated for CF3Cl and CCl4.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Bond Dissociation Energy
Bond dissociation energy (BDE) is the energy required to break a bond in a molecule, resulting in the formation of separate atoms or radicals. It is a crucial factor in determining the stability of a molecule; higher BDE indicates a stronger bond. In the context of the question, the differing BDEs of C-Cl bonds in CF3Cl and CCl4 suggest that different amounts of energy (and thus different photon wavelengths) are needed to break these bonds.
Recommended video:
Guided course
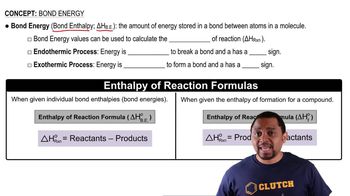
Bond Energy
Photon Energy and Wavelength
The energy of a photon is inversely related to its wavelength, described by the equation E = hc/λ, where E is energy, h is Planck's constant, c is the speed of light, and λ is the wavelength. To cause bond rupture, the energy of the photon must match or exceed the bond dissociation energy. Therefore, the range of wavelengths that can cause C-Cl bond rupture in CF3Cl and CCl4 can be calculated based on their respective BDEs.
Recommended video:
Guided course
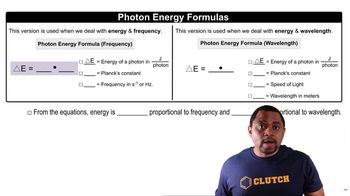
Photon Energy Formulas
Energy Threshold for Bond Rupture
The energy threshold for bond rupture is the minimum energy required to break a specific bond in a molecule. In this scenario, the C-Cl bond in CF3Cl has a higher energy threshold (339 kJ/mol) compared to CCl4 (293 kJ/mol). This difference means that photons with energies corresponding to wavelengths shorter than those calculated from these BDEs will be able to break the C-Cl bond in CF3Cl but not in CCl4, establishing a range of wavelengths for each molecule.
Recommended video:
Guided course

Bond Energy
Related Practice
Textbook Question
2
views
Textbook Question
The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (b) Which kind of electromagnetic radiation—ultraviolet, visible, or infrared—does the wavelength you calculated in part (a) correspond to?
Textbook Question
(a) Distinguish between photodissociation and photoionization.
Textbook Question
(b) Use the energy requirements of these two pro- cesses to explain why photodissociation of oxygen is more important than photoionization of oxygen at altitudes below about 90 km.
1
views
Textbook Question
The wavelength at which the O2 molecule most strongly absorbs light is approximately 145 nm. (a) In which region of the electromagnetic spectrum does this light fall?
2
views
