(a) Distinguish between photodissociation and photoionization.

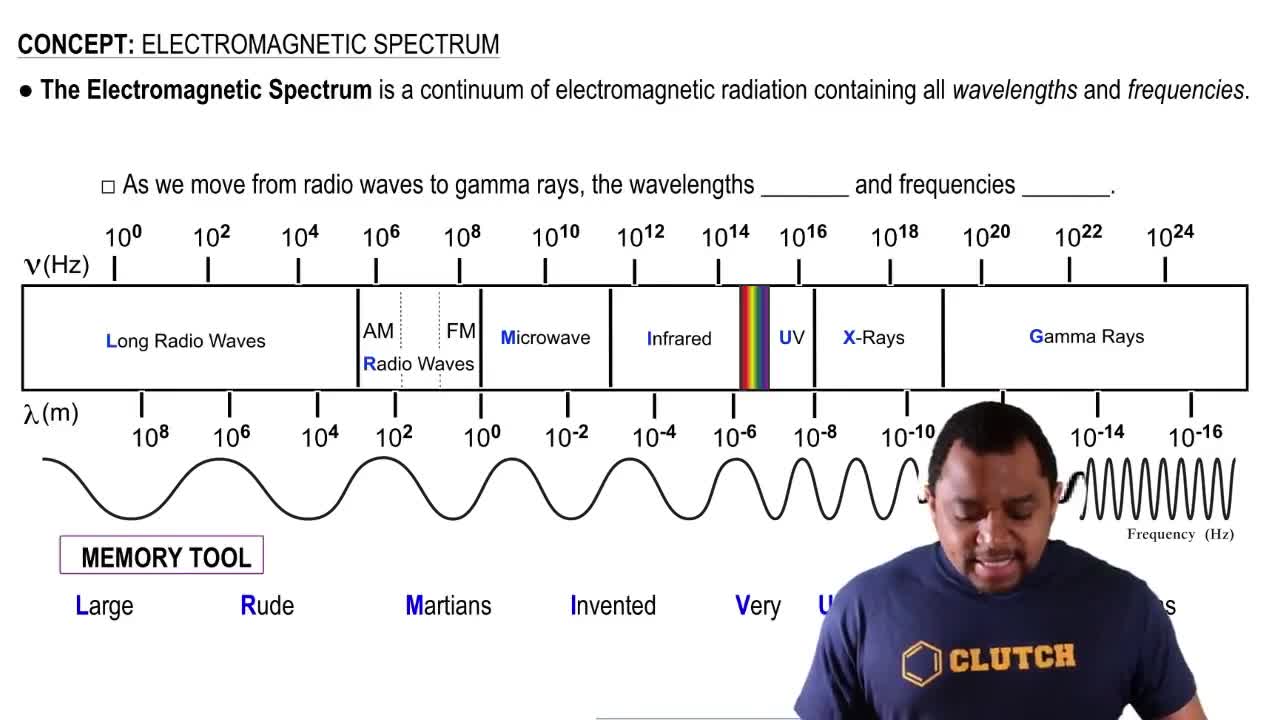
The wavelength at which the O2 molecule most strongly absorbs light is approximately 145 nm. (a) In which region of the electromagnetic spectrum does this light fall?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Electromagnetic Spectrum
Ultraviolet Light

Molecular Absorption
(b) Use the energy requirements of these two pro- cesses to explain why photodissociation of oxygen is more important than photoionization of oxygen at altitudes below about 90 km.
The wavelength at which the O2 molecule most strongly absorbs light is approximately 145 nm. (b) Would a photon whose wavelength is 145 nm have enough energy to photodissociate O2 whose bond energy is 495 kJ/mol? Would it have enough energy to photoionize O2?
The ultraviolet spectrum can be divided into three regions based on wavelength: UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm). (a) Photons from which region have the highest energy and therefore are the most harmful to living tissue? (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm).
The ultraviolet spectrum can be divided into three regions based on wavelength: UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm). (b) In the absence of ozone, which of these three regions, if any, are absorbed by the atmo- sphere?
