Textbook Question
The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (a) What is the maximum wavelength of photons that can cause C-Br bond dissociation?
2
views

 Verified step by step guidance
Verified step by step guidance
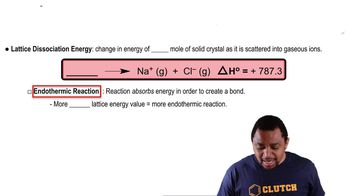
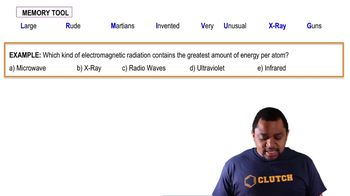

The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (a) What is the maximum wavelength of photons that can cause C-Br bond dissociation?
(a) Distinguish between photodissociation and photoionization.
(b) Use the energy requirements of these two pro- cesses to explain why photodissociation of oxygen is more important than photoionization of oxygen at altitudes below about 90 km.