Textbook Question
How would you expect the extent of overlap of the bondingatomic orbitals to vary in the series IF, ICl, IBr, and I2?Explain your answer.
2
views

 Verified step by step guidance
Verified step by step guidance


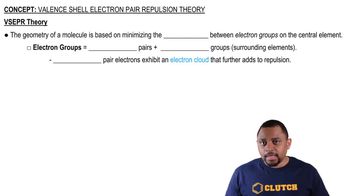
Consider the molecule BF3. (a) What is the electron configuration of an isolated B atom? (b) What is the electron configuration of an isolated F atom?
Consider the molecule BF3. (c) What hybrid orbitals should be constructed on the B atom to make the B–F bonds in BF3?
Consider the SCl2 molecule.(d) What valence orbitals, if any, remain unhybridized on the S atom in SCl2?
Indicate the hybridization of the central atom in (a) H2S (b) SeF6
Indicate the hybridization of the central atom in (c) P1OH23 (d) AlI3.