Draw sketches illustrating the overlap between the following orbitals on two atoms: (a) the 2s orbital on each atom
Ch.9 - Molecular Geometry and Bonding Theories

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 9, Problem 48
How would you expect the extent of overlap of the bondingatomic orbitals to vary in the series IF, ICl, IBr, and I2?Explain your answer.
 Verified step by step guidance
Verified step by step guidance1
Identify the concept of atomic orbital overlap: The extent of overlap between atomic orbitals is crucial for bond strength. Greater overlap generally leads to stronger bonds.
Consider the size of the halogen atoms: As you move down the halogen group in the periodic table (F, Cl, Br, I), the atomic size increases.
Analyze the effect of atomic size on overlap: Larger atoms have more diffuse orbitals, which can lead to less effective overlap compared to smaller atoms.
Compare the pairs: In the series IF, ICl, IBr, and I2, the overlap is expected to be greatest in IF and least in I2, as fluorine is the smallest atom and iodine is the largest.
Conclude with the trend: The extent of overlap decreases as the size of the halogen atom bonded to iodine increases, leading to weaker bonds in the order IF > ICl > IBr > I2.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Atomic Orbitals
Atomic orbitals are regions in an atom where there is a high probability of finding electrons. They come in different shapes (s, p, d, f) and sizes, which influence how atoms bond with each other. The overlap of atomic orbitals between two atoms is crucial for the formation of covalent bonds, as greater overlap typically leads to stronger bonds.
Recommended video:
Guided course
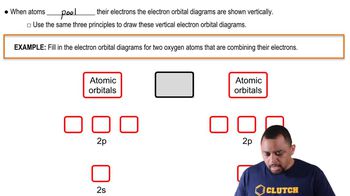
Atomic Orbitals Example
Bonding and Overlap
The extent of overlap between atomic orbitals is a key factor in determining the strength and stability of a covalent bond. When two atomic orbitals overlap significantly, they can share electrons more effectively, resulting in a stronger bond. In the context of the given compounds, the size and electronegativity of the atoms involved will affect how well their orbitals can overlap.
Recommended video:
Guided course
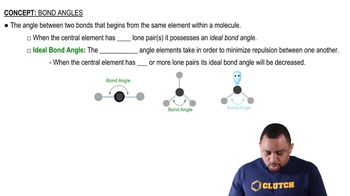
Bond Angles
Electronegativity and Bonding Characteristics
Electronegativity is a measure of an atom's ability to attract and hold onto electrons. In the series IF, ICl, IBr, and I2, the differences in electronegativity between iodine and the other halogens (F, Cl, Br) will influence the nature of the bonds formed. Higher electronegativity differences can lead to more polar bonds, affecting the overlap of bonding orbitals and the overall bond strength.
Recommended video:
Guided course

Electronegativity Trends
Related Practice
Textbook Question
Textbook Question
Draw sketches illustrating the overlap between the following orbitals on two atoms: (b) the 2pz orbital on each atom (assume both atoms are on the z-axis) (c) the 2s orbital on one atom and the 2pz orbital on the other atom.
2
views
1
rank
Textbook Question
For each statement, indicate whether it is true or false. (d) Nonbonding electron pairs cannot occupy a hybrid orbital.
Textbook Question
Consider the molecule BF3. (a) What is the electron configuration of an isolated B atom? (b) What is the electron configuration of an isolated F atom?
Textbook Question
Consider the molecule BF3. (c) What hybrid orbitals should be constructed on the B atom to make the B–F bonds in BF3?
Textbook Question
Consider the SCl2 molecule. (c) What hybrid orbitals should be constructed on the S atom to make the S-Cl bonds in SCl2?
