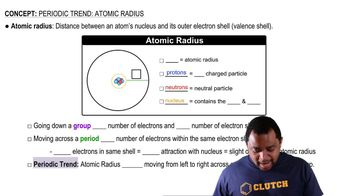
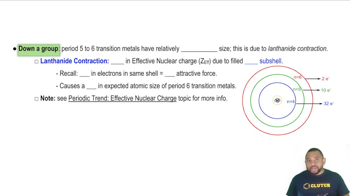
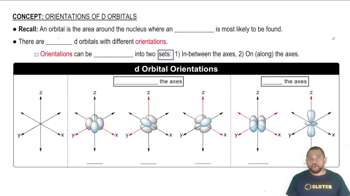
Textbook Question
The following observations are made about two hypotheticalelements A and B: The A¬A and B¬B bond lengths in theelemental forms of A and B are 236 and 194 pm, respectively.A and B react to form the binary compound AB2, which hasa linear structure (that is B-A-B = 180°). Based on thesestatements, predict the separation between the two B nucleiin a molecule of AB2.