Textbook Question
The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (b) What bond length is predicted for AsCl3, using the atomic radii in Figure 7.7?

 Verified step by step guidance
Verified step by step guidance

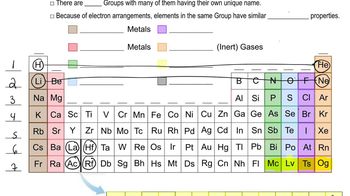

The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (b) What bond length is predicted for AsCl3, using the atomic radii in Figure 7.7?
(c) Will the lithium cobalt oxide cathode expand or contract as lithium ions are inserted?