One of the emission lines of the hydrogen atom has a wavelength of 94.974 nm. (a) In what region of the electromagnetic spectrum is this emission found?
Ch.6 - Electronic Structure of Atoms

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 6, Problem 44b
The hydrogen atom can absorb light of wavelength 1094 nm. (b) Determine the final value of n associated with this absorption.
 Verified step by step guidance
Verified step by step guidance1
Identify the formula to use: The Rydberg formula for hydrogen absorption is \( \frac{1}{\lambda} = R_H \left( \frac{1}{n_1^2} - \frac{1}{n_2^2} \right) \), where \( \lambda \) is the wavelength, \( R_H \) is the Rydberg constant (1.097 \times 10^7 \text{ m}^{-1}), \( n_1 \) is the initial energy level, and \( n_2 \) is the final energy level.
Convert the wavelength from nanometers to meters: Since 1 nm = 1 \times 10^{-9} m, convert 1094 nm to meters.
Assume the initial energy level \( n_1 \) is 1, as the problem does not specify it, and this is a common assumption for absorption from the ground state.
Rearrange the Rydberg formula to solve for \( n_2 \): \( n_2 = \sqrt{\frac{1}{\frac{1}{n_1^2} - \frac{\lambda}{R_H}}} \).
Substitute the known values into the equation: Use the converted wavelength and the Rydberg constant to calculate \( n_2 \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Energy of Photons
The energy of a photon is directly related to its wavelength, described by the equation E = hc/λ, where E is energy, h is Planck's constant, c is the speed of light, and λ is the wavelength. For the hydrogen atom, when it absorbs a photon, the energy from the photon is used to excite an electron to a higher energy level.
Recommended video:
Guided course
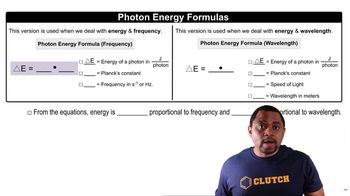
Photon Energy Formulas
Quantum Energy Levels
In a hydrogen atom, electrons occupy discrete energy levels, denoted by the principal quantum number n. The energy associated with each level can be calculated using the formula E_n = -13.6 eV/n². When a photon is absorbed, the electron transitions from a lower energy level (n_initial) to a higher one (n_final), and the difference in energy corresponds to the energy of the absorbed photon.
Recommended video:
Guided course
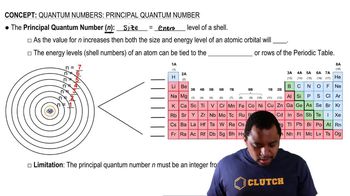
Principal Quantum Number
Balmer and Rydberg Formulas
The Rydberg formula allows for the calculation of the wavelengths of spectral lines in hydrogen and is given by 1/λ = R_H(1/n_final² - 1/n_initial²). This formula is essential for determining the final energy level (n_final) after absorption, as it relates the wavelength of light absorbed to the transition between quantum states of the hydrogen atom.
Recommended video:
Guided course

Balmer Series Example
Related Practice
Textbook Question
2
views
Textbook Question
One of the emission lines of the hydrogen atom has a wavelength of 94.974 nm. (b) Determine the initial and final values of n associated with this emission.
1
views
Textbook Question
The hydrogen atom can absorb light of wavelength 1094 nm. (a) In what region of the electromagnetic spectrum is this absorption found?
Textbook Question
Order the following transitions in the hydrogen atom from smallest to largest frequency of light absorbed: n = 3 to n = 7, n = 4 to n = 8, n = 2 to n = 5, and n = 1 to n = 3.
1
views
Textbook Question
Write the electron configurations for the following ions, anddetermine which have noble-gas configurations:(a) Ti2+(b) Br-(c) Mg2+(d) Po2-(e) Pt2+(f) V3+
Textbook Question
Use the de Broglie relationship to determine the wavelengths of the following objects: (a) an 85-kg person skiing at 50 km/hr (b) a 10.0-g bullet fired at 250 m/s
