The Lyman series of emission lines of the hydrogen atom are those for which nf = 1. (a) Determine the region of the electromagnetic spectrum in which the lines of the Lyman series are observed.

One of the emission lines of the hydrogen atom has a wavelength of 94.974 nm. (b) Determine the initial and final values of n associated with this emission.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Hydrogen Emission Spectrum
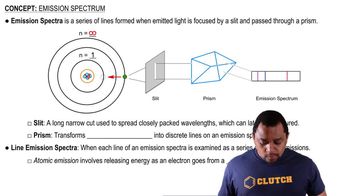
Energy Level Transitions
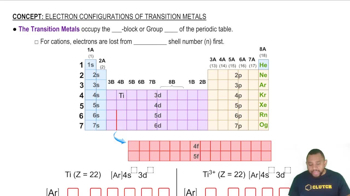
Rydberg Formula
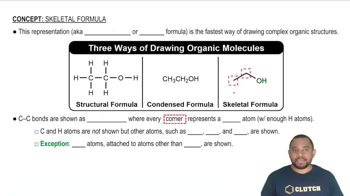
The Lyman series of emission lines of the hydrogen atom are those for which nf = 1. (b) Calculate the wavelengths of the first three lines in the Lyman series—those for which ni = 2, 3, and 4.
One of the emission lines of the hydrogen atom has a wavelength of 94.974 nm. (a) In what region of the electromagnetic spectrum is this emission found?
The hydrogen atom can absorb light of wavelength 1094 nm. (a) In what region of the electromagnetic spectrum is this absorption found?
The hydrogen atom can absorb light of wavelength 1094 nm. (b) Determine the final value of n associated with this absorption.
Order the following transitions in the hydrogen atom from smallest to largest frequency of light absorbed: n = 3 to n = 7, n = 4 to n = 8, n = 2 to n = 5, and n = 1 to n = 3.
