Textbook Question
Ethanol (C2H5OH) is blended with gasoline as an automobile fuel. (c) Calculate the heat produced per liter of ethanol by combustion of ethanol under constant pressure. Ethanol has a density of 0.789 g/mL.

 Verified step by step guidance
Verified step by step guidance
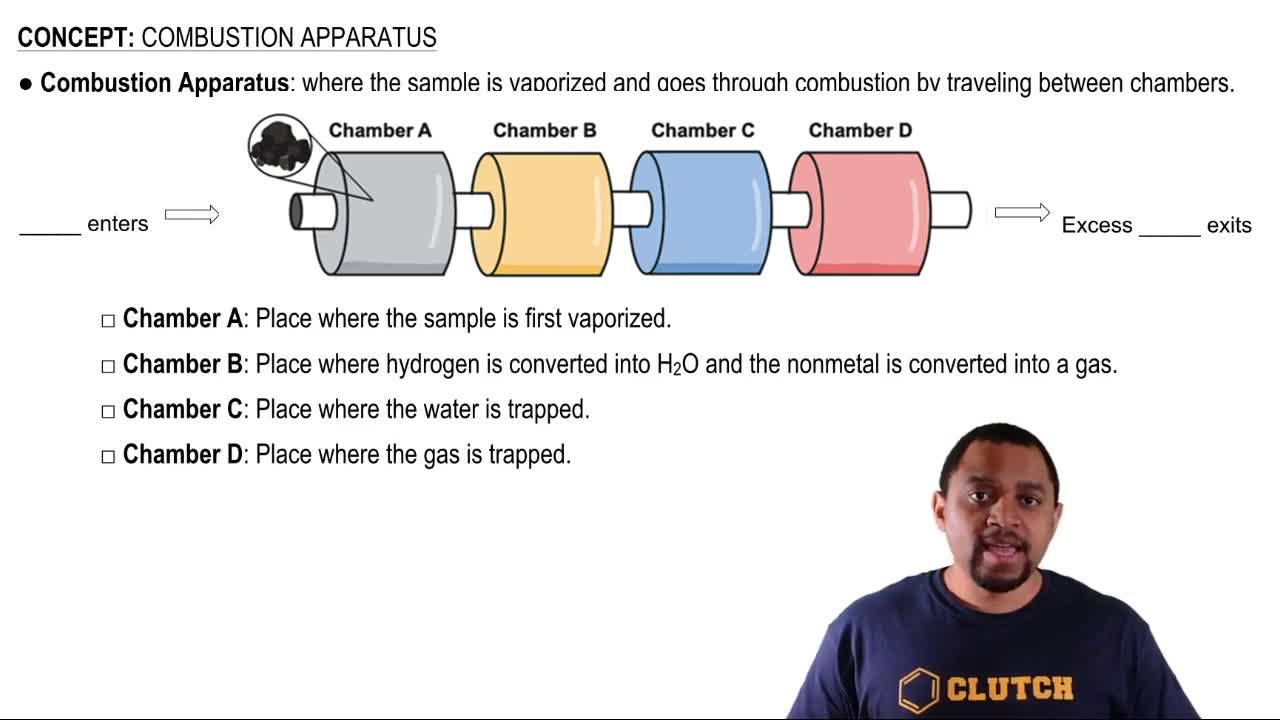

Ethanol (C2H5OH) is blended with gasoline as an automobile fuel. (c) Calculate the heat produced per liter of ethanol by combustion of ethanol under constant pressure. Ethanol has a density of 0.789 g/mL.
Ethanol (C2H5OH) is blended with gasoline as an automobile fuel. (d) Calculate the mass of CO2 produced per kJ of heat emitted.
Methanol (CH3OH) is used as a fuel in race cars. (b) Calculate the standard enthalpy change for the reaction, assuming H2O(g) as a product.
Methanol (CH3OH) is used as a fuel in race cars. (d) Calculate the mass of CO2 produced per kJ of heat emitted.