Gasoline is composed primarily of hydrocarbons, including many with eight carbon atoms, called octanes. One of the cleanest–burning octanes is a compound called 2,3,4- trimethylpentane, which has the following structural formula: The complete combustion of one mole of this compound to CO2(g) and H2O(g) leads to ΔH° = -5064.9 kJ. (b) By using the information in this problem and data in Table 5.3, calculate H°f for 2,3,4-trimethylpentane.

Ethanol (C2H5OH) is blended with gasoline as an automobile fuel. (c) Calculate the heat produced per liter of ethanol by combustion of ethanol under constant pressure. Ethanol has a density of 0.789 g/mL.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Combustion Reaction

Heat of Combustion

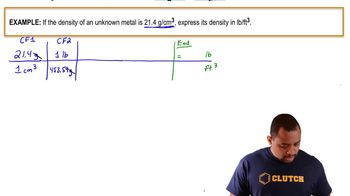
Density and Volume Conversion
Diethyl ether, C4H10O(l), a flammable compound that was once used as a surgical anesthetic, has the structure The complete combustion of 1 mol of C4H10O(l) to CO2(g) and H2O(l) yields ΔH° = -2723.7 kJ. (a) Write a balanced equation for the combustion of 1 mol of C4H10O(l).
Ethanol (C2H5OH) is blended with gasoline as an automobile fuel. (b) Calculate the standard enthalpy change for the reaction, assuming H2O(g) as a product.
Ethanol (C2H5OH) is blended with gasoline as an automobile fuel. (d) Calculate the mass of CO2 produced per kJ of heat emitted.
Methanol (CH3OH) is used as a fuel in race cars. (b) Calculate the standard enthalpy change for the reaction, assuming H2O(g) as a product.
Methanol (CH3OH) is used as a fuel in race cars. (c) Calculate the heat produced by combustion per liter of methanol. Methanol has a density of 0.791 g/mL.
