Ch.23 - Transition Metals and Coordination Chemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 23, Problem 86
Complete the exercises below. The red color of ruby is due to the presence of Cr(III) ions at octahedral sites in the close-packed oxide lattice of Al₂O₃. Draw the crystal-field splitting diagram for Cr(III) in this environment. Suppose that the ruby crystal is subjected to high pressure. What do you predict for the variation in the wavelength of absorption of the ruby as a function of pressure? Explain.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of crystal-field splitting. In an octahedral environment, the d-orbitals of a transition metal ion like Cr(III) split into two sets: the lower-energy t2g set and the higher-energy eg set. This splitting is due to the electrostatic interactions between the d-orbitals and the surrounding ligands.
Step 2: Draw the crystal-field splitting diagram for Cr(III) in an octahedral field. Start by drawing a horizontal line to represent the energy level of the five degenerate d-orbitals in a free ion. Then, split these into two groups: three orbitals (t2g) at a lower energy level and two orbitals (eg) at a higher energy level.
Step 3: Consider the effect of high pressure on the crystal-field splitting. High pressure can cause the ligands to move closer to the metal ion, increasing the electrostatic interactions and thus increasing the crystal-field splitting energy (Δ).
Step 4: Predict the effect of increased crystal-field splitting on the wavelength of absorption. According to the relationship between energy and wavelength (E = hc/λ), an increase in the crystal-field splitting energy (Δ) will result in a decrease in the wavelength (λ) of light absorbed.
Step 5: Conclude that as pressure increases, the wavelength of absorption decreases, leading to a shift in the absorption spectrum towards the blue end of the spectrum (a blue shift). This is because the energy difference between the t2g and eg orbitals increases, requiring higher energy (shorter wavelength) light to promote an electron from t2g to eg.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Crystal Field Theory
Crystal Field Theory (CFT) explains how the arrangement of ligands around a central metal ion affects its electronic energy levels. In octahedral coordination, the d-orbitals split into two energy levels: the lower-energy t2g and the higher-energy eg orbitals. This splitting is crucial for understanding the color and absorption properties of transition metal complexes, such as Cr(III) in ruby.
Recommended video:
Guided course

The study of ligand-metal interactions helped to form Ligand Field Theory which combines CFT with MO Theory.
Ligand Field Strength
The strength of the ligands surrounding a metal ion influences the extent of d-orbital splitting. Strong field ligands cause greater splitting, leading to larger energy differences between the t2g and eg levels. In the case of Cr(III) in ruby, the presence of oxide ions contributes to the ligand field strength, affecting the wavelength of light absorbed and thus the color observed.
Recommended video:
Guided course
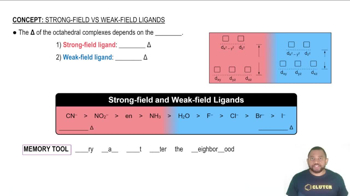
Strong-Field Ligands result in a large Δ and Weak-Field Ligands result in a small Δ.
Pressure Effects on Crystal Lattices
Applying pressure to a crystal lattice can alter the distances between ions and the overall geometry of the lattice. In the case of ruby, increased pressure may lead to changes in the crystal field splitting due to altered ligand interactions, which can shift the wavelength of absorption. Typically, higher pressure can increase the energy gap between split d-orbitals, resulting in a shift to shorter wavelengths (blue shift) in the absorption spectrum.
Recommended video:
Guided course
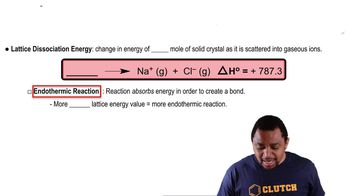
Lattice Energy
Related Practice
