Ch.23 - Transition Metals and Coordination Chemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 23, Problem 85
Complete the exercises below. Given the colors observed for VO₄³⁻ (orthovanadate ion), CrO₄²⁻ (chromate ion), and MnO₄⁻ (permanganate ion) (see Exercise 23.84), what can you say about how the energy separation between the ligand orbitals and the empty d orbitals changes as a function of the oxidation state of the transition metal at the center of the tetrahedral anion?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the oxidation states of the transition metals in each of the given ions: VO₄³⁻, CrO₄²⁻, and MnO₄⁻. For VO₄³⁻, vanadium is in the +5 oxidation state. For CrO₄²⁻, chromium is in the +6 oxidation state. For MnO₄⁻, manganese is in the +7 oxidation state.
Step 2: Understand the concept of ligand field theory, which explains the splitting of d orbitals in transition metal complexes. In a tetrahedral complex, the d orbitals split into two sets with different energies due to the presence of ligands.
Step 3: Recognize that the color observed in these ions is due to the absorption of light, which promotes an electron from a lower energy d orbital to a higher energy d orbital. The energy of the absorbed light corresponds to the energy difference between these orbitals.
Step 4: Analyze how the oxidation state of the metal affects the energy separation (Δ) between the d orbitals. Higher oxidation states generally lead to greater splitting because the metal ion has a higher positive charge, attracting the ligands more strongly and increasing the energy difference.
Step 5: Conclude that as the oxidation state of the transition metal increases from VO₄³⁻ to MnO₄⁻, the energy separation between the ligand orbitals and the empty d orbitals increases, resulting in the absorption of higher energy (shorter wavelength) light, which affects the color observed.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ligand Field Theory
Ligand Field Theory explains how the presence of ligands around a transition metal ion affects the energy levels of its d orbitals. In tetrahedral complexes, the d orbitals split into two sets with different energy levels due to the electrostatic interactions between the ligands and the metal ion. Understanding this splitting is crucial for analyzing the colors observed in the complexes, as the energy difference corresponds to the wavelengths of light absorbed.
Recommended video:
Guided course
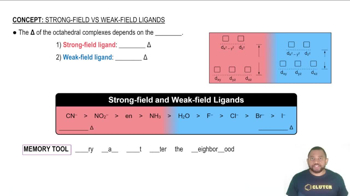
Strong-Field Ligands result in a large Δ and Weak-Field Ligands result in a small Δ.
Oxidation State and d Orbital Energy
The oxidation state of a transition metal influences the energy of its d orbitals. As the oxidation state increases, the effective nuclear charge experienced by the d electrons also increases, leading to a greater energy separation between the ligand orbitals and the d orbitals. This change can affect the color of the complex, as it alters the wavelengths of light absorbed and emitted.
Recommended video:
Guided course
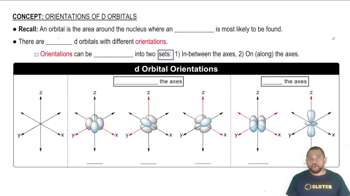
d Orbital Orientations
Color and Absorption Spectra
The color observed in transition metal complexes is a result of the specific wavelengths of light absorbed by the electrons transitioning between different energy levels. The absorption spectra of these complexes can provide insights into the energy separation between the ligand orbitals and the d orbitals. By analyzing the colors of VO₄³⁻, CrO₄²⁻, and MnO₄⁻, one can infer how the oxidation state affects these energy separations and, consequently, the observed colors.
Recommended video:
Guided course
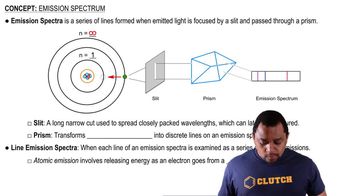
Emission Spectra
Related Practice
