Ch.23 - Transition Metals and Coordination Chemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 23, Problem 84
Complete the exercises below. Consider the tetrahedral anions VO₄³⁻ (orthovanadate ion), CrO₄²⁻ (chromate ion), and MnO₄⁻ (permanganate ion). a. These anions are isoelectronic. What does this statement mean? b. Would you expect these anions to exhibit d-d transitions? Explain. c. As mentioned in “A Closer Look” on charge-transfer color, the violet color of MnO₄⁻ is due to a ligand-to-metal charge transfer (LMCT) transition. What is meant by this term? d. The LMCT transition in MnO₄⁻ occurs at a wavelength of 565 nm. The CrO₄²⁻ ion is yellow. Is the wavelength of the LMCT transition for chromate larger or smaller than that for MnO₄⁻? Explain. e. The VO₄³⁻ ion is colorless. Do you expect the light absorbed by the LMCT to fall in the UV or the IR region of the electromagnetic spectrum? Explain your reasoning.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the term 'isoelectronic'. Isoelectronic species have the same number of electrons. For VO₄³⁻, CrO₄²⁻, and MnO₄⁻, calculate the total number of electrons in each anion to confirm they are isoelectronic.
Step 2: Consider the possibility of d-d transitions. d-d transitions occur in transition metal complexes where electrons are excited between d-orbitals. Analyze the electronic configuration of the central metal ions in VO₄³⁻, CrO₄²⁻, and MnO₄⁻ to determine if d-d transitions are possible.
Step 3: Define 'ligand-to-metal charge transfer (LMCT)'. LMCT involves the transfer of an electron from a ligand to a metal ion, often resulting in color. Explain how this process contributes to the violet color of MnO₄⁻.
Step 4: Compare the wavelengths of LMCT transitions. The color of a compound is related to the wavelength of light absorbed. Use the color of CrO₄²⁻ (yellow) and the known wavelength for MnO₄⁻ (565 nm) to infer whether the LMCT transition wavelength for CrO₄²⁻ is larger or smaller.
Step 5: Predict the region of the electromagnetic spectrum for VO₄³⁻. Since VO₄³⁻ is colorless, it does not absorb visible light. Determine whether the LMCT transition for VO₄³⁻ falls in the UV or IR region based on its lack of visible color.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Isoelectronic Species
Isoelectronic species are atoms, ions, or molecules that have the same number of electrons and, therefore, the same electronic structure. In the context of the tetrahedral anions VO₄³⁻, CrO₄²⁻, and MnO₄⁻, being isoelectronic means they all possess the same total electron count, which influences their chemical properties and reactivity. This concept is crucial for understanding how these anions behave similarly in various chemical contexts.
Recommended video:
Guided course
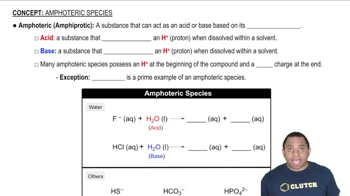
Amphoteric Species
d-d Transitions
d-d transitions refer to electronic transitions between d-orbitals in transition metal complexes. These transitions can occur when light is absorbed, promoting an electron from a lower-energy d-orbital to a higher-energy d-orbital. The presence of d-d transitions is influenced by the metal's oxidation state and the ligand field, which affects the energy levels of the d-orbitals, thus playing a significant role in the color and spectral properties of the complexes.
Recommended video:
Guided course
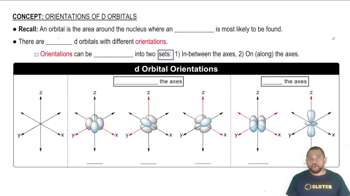
d Orbital Orientations
Ligand-to-Metal Charge Transfer (LMCT)
Ligand-to-metal charge transfer (LMCT) is a process where an electron is transferred from a ligand to a metal center in a coordination complex. This transition can result in the absorption of light, leading to color in the compound. In the case of MnO₄⁻, the violet color arises from such a transition, indicating the interaction between the ligands and the metal ion, which is essential for understanding the electronic properties and color of transition metal complexes.
Recommended video:
Guided course
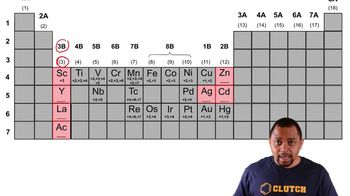
Transition Metal Element Charges
Related Practice
