Disulfides are compounds that have S ¬ S bonds, like peroxides have O ¬ O bonds. Thiols are organic compounds that have the general formula R ¬ SH, where R is a generic hydrocarbon. The SH- ion is the sulfur counterpart of hydroxide, OH-. Two thiols can react to make a disulfide, R ¬ S ¬ S ¬ R. (b) What is the oxidation state of sulfur in a disulfide?

Magnesium is obtained by electrolysis of molten MgCl2. (b) Several cells are connected in parallel by very large copper bars that convey current to the cells. Assuming that the cells are 96% efficient in producing the desired products in electrolysis, what mass of Mg is formed by passing a current of 97,000 A for a period of 24 h?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Electrolysis

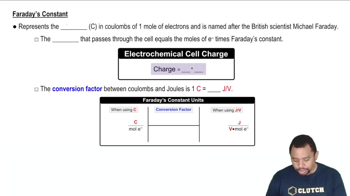
Faraday's Laws of Electrolysis
Efficiency in Electrolysis

Disulfides are compounds that have S ¬ S bonds, like peroxides have O ¬ O bonds. Thiols are organic compounds that have the general formula R ¬ SH, where R is a generic hydrocarbon. The SH- ion is the sulfur counterpart of hydroxide, OH-. Two thiols can react to make a disulfide, R ¬ S ¬ S ¬ R. (c) If you react two thiols to make a disulfide, are you oxidizing or reducing the thiols?
Calculate the number of kilowatt-hours of electricity required to produce 1.0 * 103 kg (1 metric ton) of aluminum by electrolysis of Al3+ if the applied voltage is 4.50 V and the process is 45% efficient.
The Haber process is the principal industrial route for converting nitrogen into ammonia: N2(g) + 3 H2(g) → 2 NH3(g) (b) Using the thermodynamic data in Appendix C, calculate the equilibrium constant for the process at room temperature.
