Disulfides are compounds that have S ¬ S bonds, like peroxides have O ¬ O bonds. Thiols are organic compounds that have the general formula R ¬ SH, where R is a generic hydrocarbon. The SH- ion is the sulfur counterpart of hydroxide, OH-. Two thiols can react to make a disulfide, R ¬ S ¬ S ¬ R. (b) What is the oxidation state of sulfur in a disulfide?
Ch.20 - Electrochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 20, Problem 107
(a) How many coulombs are required to plate a layer of chromium metal 0.25 mm thick on an auto bumper with a total area of 0.32 m² from a solution containing CrO₄²⁻? The density of chromium metal is 7.20 g/cm³. (b) What current flow is required for this electroplating if the bumper is to be plated in 10.0 s? (c) If the external source has an emf of +6.0 V and the electrolytic cell is 65% efficient, how much electrical power is expended to electroplate the bumper?
 Verified step by step guidance
Verified step by step guidance1
Calculate the volume of chromium needed by multiplying the thickness (converted to meters) by the area of the bumper.
Use the density of chromium to convert the volume of chromium to mass.
Convert the mass of chromium to moles using the molar mass of chromium.
Determine the number of moles of electrons required using the stoichiometry of the reduction reaction: \( \text{CrO}_4^{2-} + 8e^- + 8H^+ \rightarrow \text{Cr} + 4\text{H}_2\text{O} \).
Calculate the total charge in coulombs using the number of moles of electrons and Faraday's constant (96,485 C/mol).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electroplating and Faraday's Laws
Electroplating is a process that uses electrical current to reduce cations of a desired metal from a solution onto a conductive surface. Faraday's laws of electrolysis state that the amount of substance deposited or dissolved at an electrode is directly proportional to the quantity of electricity passed through the electrolyte. This principle is essential for calculating the total charge (in coulombs) needed to plate a specific thickness of metal.
Recommended video:
Guided course

Electroplating Process
Current and Time Relationship
The relationship between current (I), charge (Q), and time (t) is given by the equation Q = I × t. This means that the total charge required for electroplating can be determined by knowing the current flowing through the circuit and the duration of the electroplating process. Understanding this relationship is crucial for determining the current needed to achieve the desired plating thickness within a specified time.
Recommended video:
Guided course
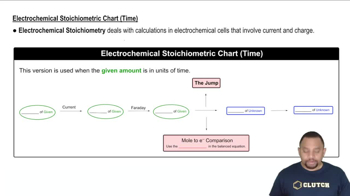
Electrochemical Stoichiometric Chart (Time)
Efficiency and Power in Electrolytic Cells
The efficiency of an electrolytic cell indicates how effectively it converts electrical energy into chemical energy for the electroplating process. In this context, if the cell operates at 65% efficiency, only 65% of the electrical power supplied is used for plating. The power (P) expended can be calculated using the formula P = IV, where I is the current and V is the voltage, adjusted for efficiency to find the actual power used in the electroplating process.
Recommended video:
Guided course

The Electrolytic Cell
Related Practice
Textbook Question
Textbook Question
Disulfides are compounds that have S ¬ S bonds, like peroxides have O ¬ O bonds. Thiols are organic compounds that have the general formula R ¬ SH, where R is a generic hydrocarbon. The SH- ion is the sulfur counterpart of hydroxide, OH-. Two thiols can react to make a disulfide, R ¬ S ¬ S ¬ R. (c) If you react two thiols to make a disulfide, are you oxidizing or reducing the thiols?
Textbook Question
Calculate the number of kilowatt-hours of electricity required to produce 1.0 * 103 kg (1 metric ton) of aluminum by electrolysis of Al3+ if the applied voltage is 4.50 V and the process is 45% efficient.
