Write the chemical formula for each substance mentioned in the following word descriptions (use the front inside cover to find the symbols for the elements you do not know). (b) On treatment with hydrofluoric acid, silicon dioxide forms silicon tetrafluoride and water. (use the front inside cover to find the symbols for the elements you do not know). (c) Sulfur dioxide reacts with water to form sulfurous acid. (use the front inside cover to find the symbols for the elements you do not know). (d) The substance phosphorus trihydride, commonly called phosphine, is a toxic gas. (e) Perchloric acid reacts with cadmium to form cadmium(II) perchlorate.
Ch.2 - Atoms, Molecules, and Ions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 2, Problem 81b2
(b) Pentane is the alkane with a chain of five carbon atoms. Determine its molecular formula.
 Verified step by step guidance
Verified step by step guidance1
Identify the general formula for alkanes, which is C_nH_{2n+2}.
Recognize that pentane has a chain of five carbon atoms, so n = 5.
Substitute n = 5 into the general formula: C_5H_{2(5)+2}.
Calculate the number of hydrogen atoms: 2(5) + 2 = 12.
Write the molecular formula for pentane as C_5H_{12}.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Alkanes
Alkanes are a class of hydrocarbons characterized by single bonds between carbon atoms. They follow the general formula CnH2n+2, where 'n' represents the number of carbon atoms. This structure results in saturated compounds, meaning they contain the maximum number of hydrogen atoms possible for a given number of carbon atoms.
Recommended video:
Guided course
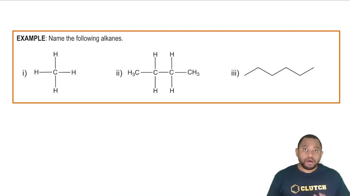
Naming Alkanes Example
Molecular Formula
The molecular formula of a compound indicates the number and types of atoms present in a molecule. For alkanes, the molecular formula can be derived from the number of carbon atoms in the chain. For pentane, which has five carbon atoms, the molecular formula can be calculated using the formula CnH2n+2.
Recommended video:
Guided course

Determining Molecular Formulas
Carbon Chain Length
The length of the carbon chain in alkanes directly influences their molecular formula and properties. In the case of pentane, the chain consists of five carbon atoms, which determines the specific arrangement and number of hydrogen atoms. Understanding the relationship between chain length and molecular formula is essential for identifying and naming alkanes.
Recommended video:
Guided course
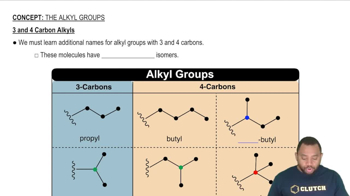
3 and 4 Carbon Alkyls
Related Practice
Textbook Question
Textbook Question
Assume that you encounter the following sentences in your reading. What is the chemical formula for each substance mentioned? (c) Hydrogen cyanide is a very poisonous gas.
Textbook Question
(a) What is a hydrocarbon?
Textbook Question
(b) Pentane is the alkane with a chain of five carbon atoms. Determine its empirical formula.
Textbook Question
(a) What is meant by the term isomer?
Textbook Question
(b) Among the four alkanes, ethane, propane, butane, and pentane, which is capable of existing in isomeric forms?
