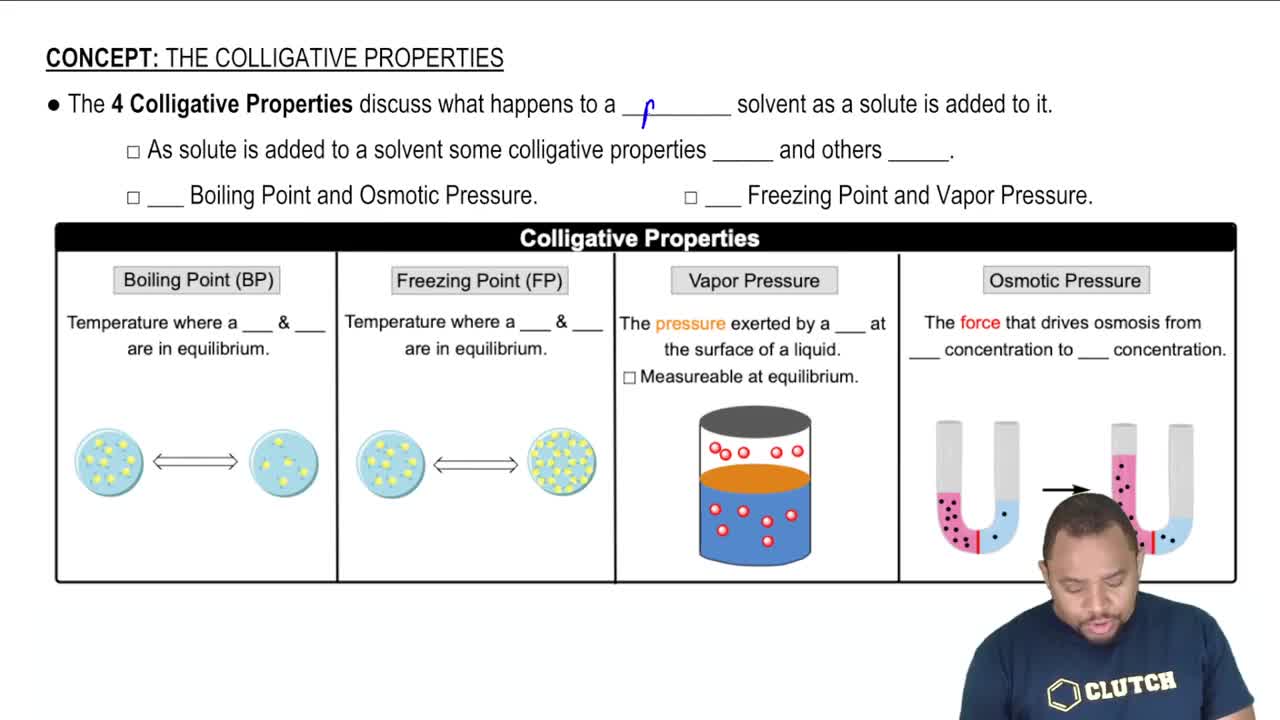
The conversion of natural gas, which is mostly methane, into products that contain two or more carbon atoms, such as ethane (C2H6), is a very important industrial chemical process. In principle, methane can be converted into ethane and hydrogen: 2 CH4(g) → C2H6(g) + H2(g) In practice, this reaction is carried out in the presence of oxygen: 2 CH4(g) + 12 O2(g) → C2H6(g) + H2O(g) (b) Is the difference in ΔG° for the two reactions due primarily to the enthalpy term (ΔH) or the entropy term (-TΔS)?