Entropy and Enthalpy
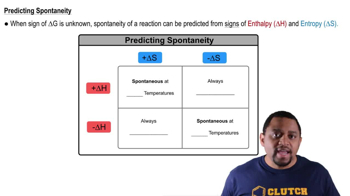
Entropy (S) is a measure of the disorder or randomness in a system, while enthalpy (H) is a measure of the total energy of a system, including internal energy and the energy required to make room for it. In the context of a reaction, an increase in entropy (positive ΔS) often favors spontaneity, especially at higher temperatures, while a decrease in enthalpy (negative ΔH) also promotes spontaneity.

 Verified step by step guidance
Verified step by step guidance