A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 17, Problem 93
A weak monoprotic acid is titrated with 0.100 M NaOH. It requires 50.0 mL of the NaOH solution to reach the equivalence point. After 25.0 mL of base is added, the pH of the solution is 3.62. Estimate the pKa of the weak acid.
 Verified step by step guidance
Verified step by step guidance1
Identify that the problem involves a weak monoprotic acid being titrated with a strong base, NaOH, and that the pH at the half-equivalence point can be used to estimate the pKa of the acid.
Recognize that at the half-equivalence point, the concentration of the weak acid equals the concentration of its conjugate base, which simplifies the Henderson-Hasselbalch equation to \( \text{pH} = \text{pKa} \).
Determine that the half-equivalence point occurs when half of the volume of NaOH required to reach the equivalence point has been added. Since 50.0 mL of NaOH is needed to reach the equivalence point, the half-equivalence point is at 25.0 mL of NaOH added.
Use the given pH of the solution after 25.0 mL of NaOH is added, which is 3.62, to estimate the pKa of the weak acid. At this point, \( \text{pH} = \text{pKa} \).
Conclude that the pKa of the weak acid is approximately equal to the pH at the half-equivalence point, which is 3.62.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Weak Acid and pKa
A weak acid is one that does not completely dissociate in solution, establishing an equilibrium between the undissociated acid and its ions. The pKa is a measure of the strength of the acid, defined as the negative logarithm of the acid dissociation constant (Ka). A lower pKa value indicates a stronger weak acid, while a higher pKa indicates a weaker acid.
Recommended video:
Guided course
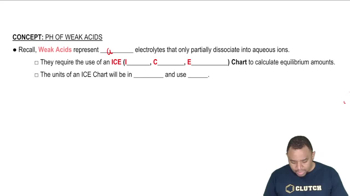
ICE Charts of Weak Acids
Titration and Equivalence Point
Titration is a quantitative analytical method used to determine the concentration of a solute in a solution. The equivalence point is reached when the amount of titrant added is stoichiometrically equivalent to the amount of substance in the sample. At this point, the moles of acid equal the moles of base, allowing for calculations related to the acid's properties.
Recommended video:
Guided course
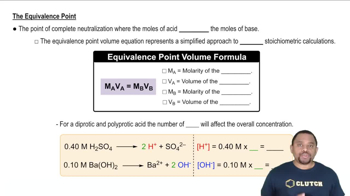
Equivalence Point in Titration
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation relates the pH of a solution to the pKa of the acid and the ratio of the concentrations of its conjugate base and the undissociated acid. It is expressed as pH = pKa + log([A-]/[HA]). This equation is particularly useful in buffer solutions and can help estimate the pKa of a weak acid when the pH and concentrations are known.
Recommended video:
Guided course

Henderson-Hasselbalch Equation
Related Practice
Textbook Question
Textbook Question
A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (b) After 7.25 mL of base had been added in the titration, the pH was found to be 2.85. What is the Ka for the unknown acid?
Textbook Question
Mathematically prove that the pH at the halfway point of a titration of a weak acid with a strong base (where the volume of added base is half of that needed to reach the equivalence point) is equal to pKa for the acid.
Textbook Question
Suppose you want to do a physiological experiment that calls for a pH 6.50 buffer. You find that the organism with which you are working is not sensitive to the weak acid H2A 1Ka1 = 2 * 10-2; Ka2 = 5.0 * 10-72 or its sodium salts. You have available a 1.0 M solution of this acid and a 1.0 M solution of NaOH. How much of the NaOH solution should be added to 1.0 L of the acid to give a buffer at pH 6.50? (Ignore any volume change.)
