Textbook Question
A sample of 0.2140 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.0950 M NaOH. The acid required 30.0 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?

 Verified step by step guidance
Verified step by step guidance
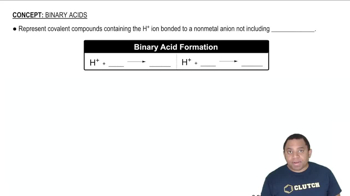
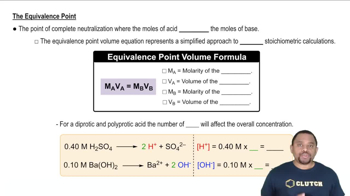
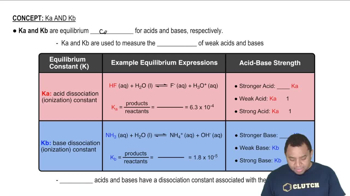
A sample of 0.2140 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.0950 M NaOH. The acid required 30.0 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
A sample of 0.1687 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.1150 M NaOH. The acid required 15.5 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?