Textbook Question
An unknown salt is either KBr, NH4Cl, KCN, or K2CO3. If a 0.100 M solution of the salt is neutral, what is the identity of the salt?

 Verified step by step guidance
Verified step by step guidance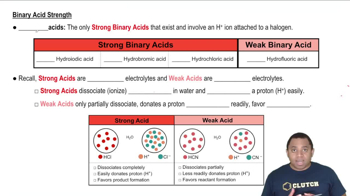
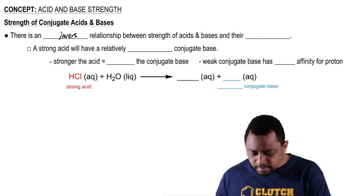
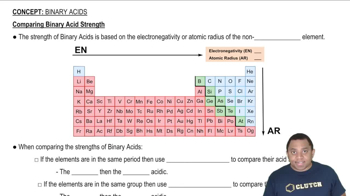
An unknown salt is either KBr, NH4Cl, KCN, or K2CO3. If a 0.100 M solution of the salt is neutral, what is the identity of the salt?
An unknown salt is either NaF, NaCl, or NaOCl. When 0.050 mol of the salt is dissolved in water to form 0.500 L of solution, the pH of the solution is 8.08. What is the identity of the salt?
Predict the stronger acid in each pair: (c) HBrO3 or HBrO2
Predict the stronger acid in each pair: (e) benzoic acid (C6H5COOH) or phenol (C6H5OH).