Textbook Question
Predict the stronger acid in each pair: (c) HBrO3 or HBrO2

 Verified step by step guidance
Verified step by step guidance
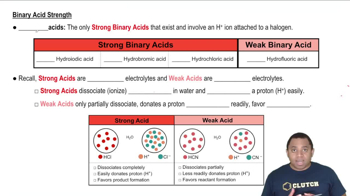


Predict the stronger acid in each pair: (c) HBrO3 or HBrO2
Based on their compositions and structures and on conjugate acid–base relationships, select the stronger base in each of the following pairs: (b) BrO- or BrO2-
Based on their compositions and structures and on conjugate acid–base relationships, select the stronger base in each of the following pairs: (b) PO43- or AsO43-