Calculate the percent ionization of propionic acid (C2H5COOH) in solutions of each of the following concentrations (Ka is given in Appendix D): (a) 0.250 M (b) 0.0800 M (c) 0.0200 M
Ch.16 - Acid-Base Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 16, Problem 67
Consider the base hydroxylamine, NH2OH. (c) There are two atoms in hydroxylamine that have nonbonding electron pairs that could act as proton acceptors. Use Lewis structures and formal charges (Section 8.5) to rationalize why one of these two atoms is a much better proton acceptor than the other.
 Verified step by step guidance
Verified step by step guidance1
Draw the Lewis structure for hydroxylamine (NH2OH). Start by counting the total number of valence electrons: nitrogen (N) has 5, each hydrogen (H) has 1, and oxygen (O) has 6, giving a total of 14 valence electrons.
Arrange the atoms with nitrogen bonded to oxygen and two hydrogens bonded to nitrogen. Distribute the remaining electrons to satisfy the octet rule for nitrogen and oxygen, ensuring that each atom has a complete valence shell.
Identify the nonbonding electron pairs: In the Lewis structure, nitrogen will have one lone pair, and oxygen will have two lone pairs. These lone pairs can act as proton acceptors.
Calculate the formal charges for nitrogen and oxygen. The formal charge is calculated using the formula: \( \text{Formal Charge} = \text{Valence Electrons} - \text{Nonbonding Electrons} - \frac{1}{2} \times \text{Bonding Electrons} \). Apply this formula to both nitrogen and oxygen to determine their formal charges.
Compare the formal charges and electronegativity of nitrogen and oxygen. Oxygen is more electronegative than nitrogen, which means it holds onto its electrons more tightly. However, the formal charge can indicate which atom is more likely to accept a proton. The atom with a lower formal charge is typically a better proton acceptor.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They help visualize the arrangement of electrons and the connectivity of atoms, allowing chemists to predict molecular geometry and reactivity. In the case of hydroxylamine, drawing its Lewis structure reveals the locations of nonbonding electron pairs on nitrogen and oxygen, which are crucial for understanding their ability to accept protons.
Recommended video:
Guided course

Lewis Dot Structures: Ions
Formal Charge
Formal charge is a theoretical charge assigned to an atom in a molecule, calculated based on the number of valence electrons, the number of nonbonding electrons, and half the number of bonding electrons. It helps determine the most stable structure of a molecule by indicating which atoms are more likely to participate in chemical reactions. In hydroxylamine, analyzing the formal charges on nitrogen and oxygen can clarify which atom is a better proton acceptor.
Recommended video:
Guided course
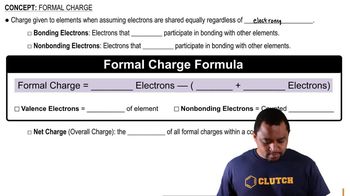
Formal Charge
Proton Acidity and Basicity
Proton acidity and basicity refer to the ability of a species to donate or accept protons (H+ ions). A stronger base has a greater tendency to accept protons, often due to the presence of lone pairs of electrons that can stabilize the added proton. In hydroxylamine, the comparison of the nonbonding electron pairs on nitrogen and oxygen will reveal which atom is more effective at accepting a proton, influencing the molecule's reactivity and properties.
Recommended video:
Guided course
Neutron-to-Proton Plot
Related Practice
Textbook Question
Textbook Question
Citric acid, which is present in citrus fruits, is a triprotic acid (Table 16.3). (a) Calculate the pH of a 0.040 M solution of citric acid. (b) Did you have to make any approximations or assumptions in completing your calculations? (c) Is the concentration of citrate ion 1C6H5O7 3-2 equal to, less than, or greater than the H+ ion concentration?
Textbook Question
Consider the base hydroxylamine, NH2OH. (a) What is the conjugate acid of hydroxylamine?
Textbook Question
The hypochlorite ion, ClO-, acts as a weak base. (a) Is ClO- a stronger or weaker base than hydroxylamine?
Textbook Question
The hypochlorite ion, ClO-, acts as a weak base. (b) When ClO- acts as a base, which atom, Cl or O, acts as the proton acceptor? (c) Can you use formal charges to rationalize your answer to part (b)?
