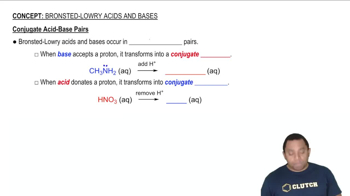
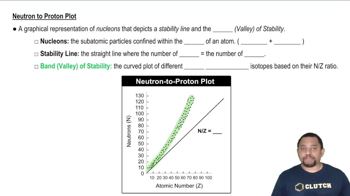
Textbook Question
Citric acid, which is present in citrus fruits, is a triprotic acid (Table 16.3). (a) Calculate the pH of a 0.040 M solution of citric acid. (b) Did you have to make any approximations or assumptions in completing your calculations? (c) Is the concentration of citrate ion 1C6H5O7 3-2 equal to, less than, or greater than the H+ ion concentration?