The reaction 2 NO(g) + O2(g) → 2 NO2 (g) is second order in NO and first order in O2. When [NO] = 0.040 M, and [O2] = 0.035 M, the observed rate of disappearance of NO is 9.3⨉10-5 M/s. (b) What is the value of the rate constant?

You perform a series of experiments for the reaction A → B + C and find that the rate law has the form rate = k[A]^x. Determine the value of x in each of the following cases: (a) There is no rate change when [A] is tripled. (b) The rate increases by a factor of 9 when [A] is tripled. (c) When [A] is doubled, the rate increases by a factor of 8.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Rate Law
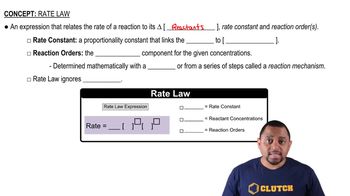
Reaction Order

Method of Initial Rates

The reaction 2 NO(g) + O2(g) → 2 NO2 (g) is second order in NO and first order in O2. When [NO] = 0.040 M, and [O2] = 0.035 M, the observed rate of disappearance of NO is 9.3⨉10-5 M/s. (c) What are the units of the rate constant?
The reaction 2 NO(g) + O2(g) → 2 NO2 (g) is second order in NO and first order in O2. When [NO] = 0.040 M, and [O2] = 0.035 M, the observed rate of disappearance of NO is 9.3⨉10-5 M/s. (d) What would happen to the rate if the concentration of NO were increased by a factor of 1.8?
Consider the following reaction between mercury(II) chloride and oxalate ion:
2 HgCl2(aq) + C2O42-(aq) → 2 Cl-(aq) + 2 CO2(g) + Hg2Cl2(s)
The initial rate of this reaction was determined for several concentrations of HgCl2 and C2O42-, and the following rate data were obtained for the rate of disappearance of C2O42-:
Experiment [HgCl2] (M) [C2O42-] (M) Rate (M/s)
1 0.164 0.15 3.2 × 10-5
2 0.164 0.45 2.9 × 10-4
3 0.082 0.45 1.4 × 10-4
4 0.246 0.15 4.8 × 10-5
(b) What is the value of the rate constant with proper units?
(c) What is the reaction rate when the initial concentration of HgCl2 is 0.100 M and that of C2O42- is 0.25 M if the temperature is the same as that used to obtain the data shown?
The reaction 2 NO2 → 2 NO + O2 has the rate constant k = 0.63 M-1s-1. (a) Based on the units for k, is the reaction first or second order in NO2?
