Consider the reaction A + B → C + D. Is each of the following statements true or false? (b) If the reaction is an elementary reaction, the rate law is second order.
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 91b
The reaction 2 NO(g) + O2(g) → 2 NO2 (g) is second order in NO and first order in O2. When [NO] = 0.040 M, and [O2] = 0.035 M, the observed rate of disappearance of NO is 9.3⨉10-5 M/s. (b) What is the value of the rate constant?
 Verified step by step guidance
Verified step by step guidance1
Identify the rate law for the reaction. Since the reaction is second order in NO and first order in O_2, the rate law is: rate = k[NO]^2[O_2].
Substitute the given concentrations and the rate of disappearance into the rate law equation. You have: 9.3 \times 10^{-5} \text{ M/s} = k (0.040 \text{ M})^2 (0.035 \text{ M}).
Solve the equation for the rate constant k. Rearrange the equation to isolate k: k = \frac{9.3 \times 10^{-5} \text{ M/s}}{(0.040 \text{ M})^2 (0.035 \text{ M})}.
Calculate the value of k using the rearranged equation. Ensure that you perform the arithmetic operations correctly to find the numerical value of k.
Remember to include the correct units for the rate constant k. Since the reaction is third order overall (second order in NO and first order in O_2), the units for k will be M^{-2} s^{-1}.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Rate Law
The rate law expresses the relationship between the rate of a chemical reaction and the concentration of its reactants. For the given reaction, the rate law can be written as Rate = k[NO]^2[O2]^1, where k is the rate constant. Understanding the rate law is essential for determining how changes in concentration affect the reaction rate.
Recommended video:
Guided course
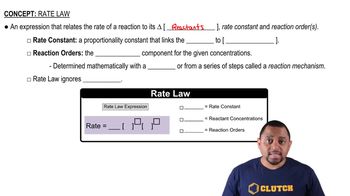
Rate Law Fundamentals
Order of Reaction
The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate law. In this case, the reaction is second order with respect to NO and first order with respect to O2. The overall order of the reaction is the sum of the individual orders, which helps in calculating the rate constant and understanding the kinetics of the reaction.
Recommended video:
Guided course

Average Bond Order
Rate Constant (k)
The rate constant (k) is a proportionality factor in the rate law that is specific to a given reaction at a certain temperature. It reflects the speed of the reaction and can be determined using the observed rate and concentrations of the reactants. Calculating k is crucial for predicting how the reaction will behave under different conditions.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
Textbook Question
Consider the reaction A + B → C + D. Is each of the following statements true or false? (c) If the reaction is an elementary reaction, the rate law of the reverse reaction is first order.
Textbook Question
The reaction 2 NO(g) + O2(g) → 2 NO2 (g) is second order in NO and first order in O2. When [NO] = 0.040 M, and [O2] = 0.035 M, the observed rate of disappearance of NO is 9.3⨉10-5 M/s. (c) What are the units of the rate constant?
Textbook Question
The reaction 2 NO(g) + O2(g) → 2 NO2 (g) is second order in NO and first order in O2. When [NO] = 0.040 M, and [O2] = 0.035 M, the observed rate of disappearance of NO is 9.3⨉10-5 M/s. (d) What would happen to the rate if the concentration of NO were increased by a factor of 1.8?
